Deletion of Endogenous Neuregulin-4 Limits Adaptive Immunity During Interleukin-10 Receptor-Neutralizing Colitis
- PMID: 37265326
- PMCID: PMC10628918
- DOI: 10.1093/ibd/izad092
Deletion of Endogenous Neuregulin-4 Limits Adaptive Immunity During Interleukin-10 Receptor-Neutralizing Colitis
Abstract
Background: Growth factors are essential for maintenance of intestinal health. We previously showed that exogenous neuregulin-4 (NRG4) promotes colonocyte survival during cytokine challenge and is protective against acute models of intestinal inflammation. However, the function(s) of endogenous NRG4 are not well understood. Using NRG4-/- mice, we tested the role of endogenous NRG4 in models of colitis skewed toward either adaptive (interleukin-10 receptor [IL-10R] neutralization) or innate (dextran sulfate sodium [DSS]) immune responses.
Methods: NRG4-/- and wild-type cage mate mice were subjected to chronic IL-10R neutralization colitis and acute DSS colitis. Disease was assessed by histological examination, inflammatory cytokine levels, fecal lipocalin-2 levels, and single cell mass cytometry immune cell profiling. Homeostatic gene alterations were evaluated by RNA sequencing analysis from colonic homogenates, with real-time quantitative polymerase chain reaction confirmation in both tissue and isolated epithelium.
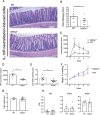
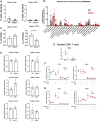
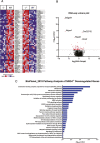
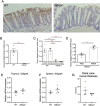
Results: During IL-10R neutralization colitis, NRG4-/- mice had reduced colonic inflammatory cytokine expression, histological damage, and colonic CD8+ T cell numbers vs wild-type cage mates. Conversely, in DSS colitis, NRG4-/- mice had elevated cytokine expression, fecal lipocalin-2 levels, and impaired weight recovery. RNA sequencing showed a loss of St3gal4, a sialyltransferase involved in immune cell trafficking, in NRG4-null colons, which was verified in both tissue and isolated epithelium. The regulation of St3gal4 by NRG4 was confirmed with ex vivo epithelial colon organoid cultures from NRG4-/- mice and by induction of St3gal4 in vivo following NRG4 treatment.
Conclusions: NRG4 regulates colonic epithelial ST3GAL4 and thus may allow for robust recruitment of CD8+ T cells during adaptive immune responses in colitis. On the other hand, NRG4 loss exacerbates injury driven by innate immune responses.
Keywords: ErbB receptor tyrosine kinases; adaptive immunity; experimental colitis; neuregulin growth factors.
Plain language summary
Neuregulin-4 (NRG4) is a growth factor that protects the epithelial cells lining the colon from injury and restrains innate (non-specific) immune responses. Here we show that NRG4’s role in inflammation is context-specific, and mice that lack NRG4 have impaired adaptive immunity in a model of chronic immune-mediated colitis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
M.R.F. and M.A.S. are inventors on a patent held by Children’s Hospital Los Angeles on the possible therapeutic use of neuregulin-4 in intestinal inflammation. The other authors have no conflicts.
Figures



Similar articles
-
Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice.Gastroenterology. 2013 Sep;145(3):591-601.e3. doi: 10.1053/j.gastro.2013.05.047. Epub 2013 May 31. Gastroenterology. 2013. PMID: 23732773 Free PMC article.
-
MTG16 contributes to colonic epithelial integrity in experimental colitis.Gut. 2013 Oct;62(10):1446-55. doi: 10.1136/gutjnl-2011-301439. Epub 2012 Jul 24. Gut. 2013. PMID: 22833394 Free PMC article.
-
Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis.World J Gastroenterol. 2017 Feb 14;23(6):999-1009. doi: 10.3748/wjg.v23.i6.999. World J Gastroenterol. 2017. PMID: 28246473 Free PMC article.
-
Lactobacillus crispatus M206119 exacerbates murine DSS-colitis by interfering with inflammatory responses.World J Gastroenterol. 2012 May 21;18(19):2344-56. doi: 10.3748/wjg.v18.i19.2344. World J Gastroenterol. 2012. PMID: 22654425 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

