Translation of the efficacy of antibody-drug conjugates from preclinical to clinical using a semimechanistic PK/PD model: A case study with RC88
- PMID: 37259689
- PMCID: PMC10339704
- DOI: 10.1111/cts.13526
Translation of the efficacy of antibody-drug conjugates from preclinical to clinical using a semimechanistic PK/PD model: A case study with RC88
Abstract
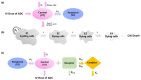
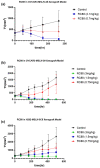
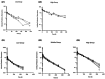
Three semimechanistic pharmacokinetic/pharmacodynamic (PK/PD) models, Simeoni, Jumbe, and Hybrid, were used for the efficacy translation of RC88 from preclinical to clinical. RC88 is a mesothelin-targeting antibody-drug conjugate for malignant solid tumor. In the preclinical study, the relationship between PKs and PDs was determined using the xenograft mouse model derived from ovarian cancer and lung cancer cell lines. A secondary parameter representing the efficacy index of the drug, termed as tumor static concentration (TSC), was calculated using the three semimechanistic PK/PD models. A mechanism-based target-mediated drug disposition model was used to predict the human PKs. TSC from mice and predicted human PK were integrated to predict human efficacy dose. Results showed that 2 cell lines were sensitive to drugs, and the predicted efficacy dose was between 0.82 and 1.96 mg/kg q1w.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
Similar articles
-
On translation of antibody drug conjugates efficacy from mouse experimental tumors to the clinic: a PK/PD approach.J Pharmacokinet Pharmacodyn. 2013 Oct;40(5):557-71. doi: 10.1007/s10928-013-9329-x. Epub 2013 Aug 10. J Pharmacokinet Pharmacodyn. 2013. PMID: 23933716
-
Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin.J Pharmacokinet Pharmacodyn. 2012 Dec;39(6):643-59. doi: 10.1007/s10928-012-9276-y. Epub 2012 Nov 15. J Pharmacokinet Pharmacodyn. 2012. PMID: 23151991
-
Preclinical safety profile of RC88-ADC:a novel mesothelin-targeted antibody conjugated with Monomethyl auristatin E.Drug Chem Toxicol. 2023 Jan;46(1):24-34. doi: 10.1080/01480545.2021.2005085. Epub 2021 Nov 28. Drug Chem Toxicol. 2023. PMID: 34839761
-
Impact of Physiologically Based Pharmacokinetics, Population Pharmacokinetics and Pharmacokinetics/Pharmacodynamics in the Development of Antibody-Drug Conjugates.J Clin Pharmacol. 2020 Oct;60 Suppl 1(Suppl 1):S105-S119. doi: 10.1002/jcph.1720. J Clin Pharmacol. 2020. PMID: 33205423 Free PMC article. Review.
-
Application of Pharmacokinetic-Pharmacodynamic Modeling and Simulation for Antibody-Drug Conjugate Development.Pharm Res. 2015 Nov;32(11):3508-25. doi: 10.1007/s11095-015-1626-1. Epub 2015 Feb 11. Pharm Res. 2015. PMID: 25666843 Review.
References
-
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS‐8201), a HER2‐targeting antibody‐drug conjugate, in patients with advanced breast and gastric or gastro‐oesophageal tumours: a phase 1 dose‐escalation study. Lancet Oncol. 2017;18:1512‐1522. doi:10.1016/S1470-2045(17)30604-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

