Molecular structure of soluble vimentin tetramers
- PMID: 37258554
- PMCID: PMC10232555
- DOI: 10.1038/s41598-023-34814-4
Molecular structure of soluble vimentin tetramers
Erratum in
-
Author Correction: Molecular structure of soluble vimentin tetramers.Sci Rep. 2024 Nov 20;14(1):28721. doi: 10.1038/s41598-024-79005-x. Sci Rep. 2024. PMID: 39567589 Free PMC article. No abstract available.
Abstract
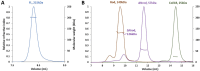
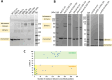
Intermediate filaments (IFs) are essential constituents of the metazoan cytoskeleton. A vast family of cytoplasmic IF proteins are capable of self-assembly from soluble tetrameric species into typical 10-12 nm wide filaments. The primary structure of these proteins includes the signature central 'rod' domain of ~ 300 residues which forms a dimeric α-helical coiled coil composed of three segments (coil1A, coil1B and coil2) interconnected by non-helical, flexible linkers (L1 and L12). The rod is flanked by flexible terminal head and tail domains. At present, the molecular architecture of mature IFs is only poorly known, limiting our capacity to rationalize the effect of numerous disease-related mutations found in IF proteins. Here we addressed the molecular structure of soluble vimentin tetramers which are formed by two antiparallel, staggered dimers with coil1B domains aligned (A11 tetramers). By examining a series of progressive truncations, we show that the presence of the coil1A domain is essential for the tetramer formation. In addition, we employed a novel chemical cross-linking pipeline including isotope labelling to identify intra- and interdimeric cross-links within the tetramer. We conclude that the tetramer is synergistically stabilized by the interactions of the aligned coil1B domains, the interactions between coil1A and the N-terminal portion of coil2, and the electrostatic attraction between the oppositely charged head and rod domains. Our cross-linking data indicate that, starting with a straight A11 tetramer, flexibility of linkers L1 and L12 enables 'backfolding' of both the coil1A and coil2 domains onto the tetrameric core formed by the coil1B domains. Through additional small-angle X-ray scattering experiments we show that the elongated A11 tetramers dominate in low ionic strength solutions, while there is also a significant structural flexibility especially in the terminal domains.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Stability profile of vimentin rod domain.Protein Sci. 2022 Dec;31(12):e4505. doi: 10.1002/pro.4505. Protein Sci. 2022. PMID: 36369679 Free PMC article.
-
Atomic structure of the vimentin central α-helical domain and its implications for intermediate filament assembly.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13620-5. doi: 10.1073/pnas.1206836109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869704 Free PMC article.
-
Lateral A11 type tetramerization in lamins.J Struct Biol. 2020 Jan 1;209(1):107404. doi: 10.1016/j.jsb.2019.10.006. Epub 2019 Oct 11. J Struct Biol. 2020. PMID: 31610238
-
Intermediate filament structure: the bottom-up approach.Curr Opin Cell Biol. 2015 Feb;32:65-72. doi: 10.1016/j.ceb.2014.12.007. Epub 2015 Jan 14. Curr Opin Cell Biol. 2015. PMID: 25596497 Review.
-
Recent insight into intermediate filament structure.Curr Opin Cell Biol. 2021 Feb;68:132-143. doi: 10.1016/j.ceb.2020.10.001. Epub 2020 Nov 12. Curr Opin Cell Biol. 2021. PMID: 33190098 Free PMC article. Review.
Cited by
-
Neurofilament Light Protein Rod Domain Exhibits Structural Heterogeneity.Biomolecules. 2024 Jan 9;14(1):85. doi: 10.3390/biom14010085. Biomolecules. 2024. PMID: 38254685 Free PMC article.
-
Structural Characterization of Monoclonal Antibodies and Epitope Mapping by FFAP Footprinting.Anal Chem. 2024 May 14;96(19):7386-7393. doi: 10.1021/acs.analchem.3c04161. Epub 2024 May 2. Anal Chem. 2024. PMID: 38698660 Free PMC article.
-
Type III intermediate filaments in redox interplay: key role of the conserved cysteine residue.Biochem Soc Trans. 2024 Apr 24;52(2):849-860. doi: 10.1042/BST20231059. Biochem Soc Trans. 2024. PMID: 38451193 Free PMC article. Review.
-
Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein-ACE2 Interactions.Int J Mol Sci. 2024 Feb 20;25(5):2477. doi: 10.3390/ijms25052477. Int J Mol Sci. 2024. PMID: 38473724 Free PMC article.
-
Vimentin filaments integrate low-complexity domains in a complex helical structure.Nat Struct Mol Biol. 2024 Jun;31(6):939-949. doi: 10.1038/s41594-024-01261-2. Epub 2024 Apr 17. Nat Struct Mol Biol. 2024. PMID: 38632361 Free PMC article.
References
-
- Kreplak, L. & Fudge, D. Biomechanical properties of intermediate filaments: From tissues to single filaments and back. BioEssays News Rev. Mol. Cell. Dev. Biol29, 26–35. 10.1002/bies.20514 (2007). - PubMed
-
- Herrmann, H., Bär, H., Kreplak, L., Strelkov, S. V. & Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol.8, 562. 10.1038/nrm2197 (2007). - PubMed
-
- Herrmann, H. & Aebi, U. Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu. Rev. Biochem.73, 749–789. 10.1146/annurev.biochem.73.011303.073823 (2004). - PubMed
-
- Chernyatina, A. A., Guzenko, D. & Strelkov, S. V. Intermediate filament structure: The bottom-up approach. Curr. Opin. Cell Biol.32, 65–72. 10.1016/j.ceb.2014.12.007 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

