Viral vector delivered immunogen focuses HIV-1 antibody specificity and increases durability of the circulating antibody recall response
- PMID: 37256916
- PMCID: PMC10284421
- DOI: 10.1371/journal.ppat.1011359
Viral vector delivered immunogen focuses HIV-1 antibody specificity and increases durability of the circulating antibody recall response
Abstract
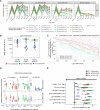
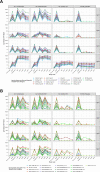
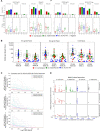
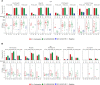
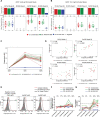
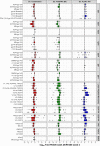
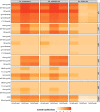
The modestly efficacious HIV-1 vaccine regimen (RV144) conferred 31% vaccine efficacy at 3 years following the four-shot immunization series, coupled with rapid waning of putative immune correlates of decreased infection risk. New strategies to increase magnitude and durability of protective immunity are critically needed. The RV305 HIV-1 clinical trial evaluated the immunological impact of a follow-up boost of HIV-1-uninfected RV144 recipients after 6-8 years with RV144 immunogens (ALVAC-HIV alone, AIDSVAX B/E gp120 alone, or ALVAC-HIV + AIDSVAX B/E gp120). Previous reports demonstrated that this regimen elicited higher binding, antibody Fc function, and cellular responses than the primary RV144 regimen. However, the impact of the canarypox viral vector in driving antibody specificity, breadth, durability and function is unknown. We performed a follow-up analysis of humoral responses elicited in RV305 to determine the impact of the different booster immunogens on HIV-1 epitope specificity, antibody subclass, isotype, and Fc effector functions. Importantly, we observed that the ALVAC vaccine component directly contributed to improved breadth, function, and durability of vaccine-elicited antibody responses. Extended boosts in RV305 increased circulating antibody concentration and coverage of heterologous HIV-1 strains by V1V2-specific antibodies above estimated protective levels observed in RV144. Antibody Fc effector functions, specifically antibody-dependent cellular cytotoxicity and phagocytosis, were boosted to higher levels than was achieved in RV144. V1V2 Env IgG3, a correlate of lower HIV-1 risk, was not increased; plasma Env IgA (specifically IgA1), a correlate of increased HIV-1 risk, was elevated. The quality of the circulating polyclonal antibody response changed with each booster immunization. Remarkably, the ALVAC-HIV booster immunogen induced antibody responses post-second boost, indicating that the viral vector immunogen can be utilized to selectively enhance immune correlates of decreased HIV-1 risk. These results reveal a complex dynamic of HIV-1 immunity post-vaccination that may require careful balancing to achieve protective immunity in the vaccinated population. Trial registration: RV305 clinical trial (ClinicalTrials.gov number, NCT01435135). ClinicalTrials.gov Identifier: NCT00223080.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial.PLoS Pathog. 2017 Feb 24;13(2):e1006182. doi: 10.1371/journal.ppat.1006182. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28235027 Free PMC article. Clinical Trial.
-
Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial.Lancet HIV. 2020 Apr;7(4):e238-e248. doi: 10.1016/S2352-3018(19)30406-0. Epub 2020 Feb 6. Lancet HIV. 2020. PMID: 32035516 Free PMC article. Clinical Trial.
-
Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations.PLoS One. 2018 Apr 27;13(4):e0196397. doi: 10.1371/journal.pone.0196397. eCollection 2018. PLoS One. 2018. PMID: 29702672 Free PMC article. Clinical Trial.
-
Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection.Annu Rev Med. 2015;66:423-37. doi: 10.1146/annurev-med-052912-123749. Epub 2014 Oct 17. Annu Rev Med. 2015. PMID: 25341006 Review.
-
Prospects for a Globally Effective HIV-1 Vaccine.Am J Prev Med. 2015 Dec;49(6 Suppl 4):S307-18. doi: 10.1016/j.amepre.2015.09.004. Am J Prev Med. 2015. PMID: 26590431 Review.
Cited by
-
CD4 downregulation precedes Env expression and protects HIV-1-infected cells from ADCC mediated by non-neutralizing antibodies.mBio. 2024 Nov 13;15(11):e0182724. doi: 10.1128/mbio.01827-24. Epub 2024 Oct 7. mBio. 2024. PMID: 39373535 Free PMC article.
-
Safety and immunogenicity after a 30-month boost of a subtype C ALVAC-HIV (vCP2438) vaccine prime plus bivalent subtype C gp120/MF59 vaccine boost (HVTN 100): A phase 1-2 randomized double-blind placebo-controlled trial.PLOS Glob Public Health. 2024 Sep 20;4(9):e0003319. doi: 10.1371/journal.pgph.0003319. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39302924 Free PMC article.
References
-
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, et al.. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. 2012;12(7):531–7. doi: 10.1016/S1473-3099(12)70088-9 ; PubMed Central PMCID: PMC3530398. - DOI - PMC - PubMed
-
- Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al.. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110(22):9019–24. doi: 10.1073/pnas.1301456110 ; PubMed Central PMCID: PMC3670311. - DOI - PMC - PubMed
-
- Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, et al.. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One. 2013;8(9):e75665. doi: 10.1371/journal.pone.0075665 ; PubMed Central PMCID: PMC3784573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

