Differential Susceptibility of Aedes aegypti and Aedes albopictus Mosquitoes to Infection by Mayaro Virus Strains
- PMID: 37253447
- PMCID: PMC10323988
- DOI: 10.4269/ajtmh.22-0777
Differential Susceptibility of Aedes aegypti and Aedes albopictus Mosquitoes to Infection by Mayaro Virus Strains
Abstract
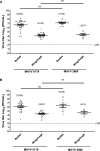
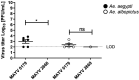
Mayaro virus (MAYV) is an arthropod-borne virus (arbovirus) belonging to the family Togaviridae, genus Alphavirus. In recent years, the geographic distribution of MAYV may have expanded north from South and Central America into the Caribbean Islands. Although Haemagogus janthinomys is considered the main vector for MAYV, the virus has also been isolated from other mosquitoes, including Aedes aegypti, a widespread species that serves as the main vector for highly epidemic viruses. Given the possible expansion and outbreaks of MAYV in Latin America, it is possible that MAYV might be adapting to be efficiently transmitted by urban vectors. Therefore, to investigate this possibility, we evaluated the vector competence of Ae. aegypti and Ae. albopictus mosquitoes to transmit MAYV isolated during a year of low or high MAYV transmission. Adult Ae. aegypti and Ae. albopictus were orally infected with the MAYV strains, and the infection, dissemination, and transmission rates were calculated to evaluate their vector competence. Overall, we found higher infection, dissemination, and transmission rates in both Ae. aegypti and Ae. albopictus mosquitoes infected with the strain isolated during a MAYV outbreak, whereas low/no transmission was detected with the strain isolated during a year of low MAYV activity. Our results confirmed that both Ae. aegypti and Ae. albopictus are competent vectors for the emergent MAYV. Our data suggest that strains isolated during MAYV outbreaks might be better fit to infect and be transmitted by urban vectors, raising serious concern about the epidemic potential of MAYV.
Figures
Similar articles
-
Vector competence of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes for Mayaro virus.PLoS Negl Trop Dis. 2020 Apr 14;14(4):e0007518. doi: 10.1371/journal.pntd.0007518. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32287269 Free PMC article.
-
Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes.Med Vet Entomol. 2018 Dec;32(4):436-442. doi: 10.1111/mve.12322. Epub 2018 Jul 13. Med Vet Entomol. 2018. PMID: 30006976
-
Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus.Viruses. 2019 Oct 9;11(10):924. doi: 10.3390/v11100924. Viruses. 2019. PMID: 31601017 Free PMC article.
-
GloPID-R report on chikungunya, o'nyong-nyong and Mayaro virus, part 5: Entomological aspects.Antiviral Res. 2020 Feb;174:104670. doi: 10.1016/j.antiviral.2019.104670. Epub 2019 Dec 5. Antiviral Res. 2020. PMID: 31812638 Review.
-
[Mayaro: a re-emerging Arbovirus in Venezuela and Latin America].Biomedica. 2012 Jun;32(2):286-302. doi: 10.1590/S0120-41572012000300017. Biomedica. 2012. PMID: 23242303 Review. Spanish.
Cited by
-
Molecular Epidemiology of Mayaro Virus among Febrile Patients, Roraima State, Brazil, 2018-2021.Emerg Infect Dis. 2024 May;30(5):1013-1016. doi: 10.3201/eid3005.231406. Emerg Infect Dis. 2024. PMID: 38666638 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

