Mycobacterium tuberculosis CitA activity is modulated by cysteine oxidation and pyruvate binding
- PMID: 37252106
- PMCID: PMC10211323
- DOI: 10.1039/d3md00058c
Mycobacterium tuberculosis CitA activity is modulated by cysteine oxidation and pyruvate binding
Abstract
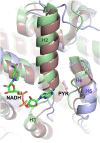
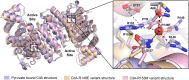
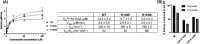
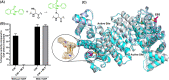
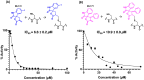
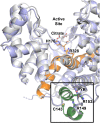
As an adaptation for survival during infection, Mycobacterium tuberculosis becomes dormant, reducing its metabolism and growth. Two types of citrate synthases have been identified in Mycobacterium tuberculosis, GltA2 and CitA. Previous work shows that overexpression of CitA, the secondary citrate synthase, stimulates the growth of Mycobacterium tuberculosis under hypoxic conditions without showing accumulation of triacylglycerols and makes mycobacteria more sensitive to antibiotics, suggesting that CitA may play a role as a metabolic switch during infection and may be an interesting TB drug target. To assess the druggability and possible mechanisms of targeting CitA with small-molecule compounds, the CitA crystal structure was solved to 2.1 Å by X-ray crystallography. The solved structure shows that CitA lacks an NADH binding site that would afford allosteric regulation, which is atypical of most citrate synthases. However, a pyruvate molecule is observed within the analogous domain, suggesting pyruvate may instead be the allosteric regulator for CitA. The R149 and R153 residues forming the charged portion of the pyruvate binding pocket were mutated to glutamate and methionine, respectively, to assess the effect of mutations on activity. Protein thermal shift assay shows thermal stabilization of CitA in the presence of pyruvate compared to the two CitA variants designed to decrease pyruvate affinity. Solved crystal structures of both variants show no significant structural changes. However, the catalytic efficiency of the R153M variant increases by 2.6-fold. Additionally, we show that covalent modification of C143 of CitA by Ebselen completely arrests enzyme activity. Similar inhibition is observed using two spirocyclic Michael acceptor containing compounds, which inhibit CitA with ICapp50 values of 6.6 and 10.9 μM. A crystal structure of CitA modified by Ebselen was solved, but significant structural changes were lacking. Considering that covalent modification of C143 inactivates CitA and the proximity of C143 to the pyruvate binding site, this suggests that structural and/or chemical changes in this sub-domain are responsible for regulating CitA enzymatic activity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare. The research presented here was not supported by nor represents experiments performed by BioNTech.
Figures

Similar articles
-
Exploring Covalent Allosteric Inhibition of Antigen 85C from Mycobacterium tuberculosis by Ebselen Derivatives.ACS Infect Dis. 2017 May 12;3(5):378-387. doi: 10.1021/acsinfecdis.7b00003. Epub 2017 Mar 21. ACS Infect Dis. 2017. PMID: 28285521 Free PMC article.
-
Inactivation of the Mycobacterium tuberculosis antigen 85 complex by covalent, allosteric inhibitors.J Biol Chem. 2014 Sep 5;289(36):25031-40. doi: 10.1074/jbc.M114.582445. Epub 2014 Jul 14. J Biol Chem. 2014. PMID: 25028518 Free PMC article.
-
Role of acid metabolism in Streptomyces coelicolor morphological differentiation and antibiotic biosynthesis.J Bacteriol. 2001 May;183(10):3184-92. doi: 10.1128/JB.183.10.3184-3192.2001. J Bacteriol. 2001. PMID: 11325948 Free PMC article.
-
Small organic molecules targeting the energy metabolism of Mycobacterium tuberculosis.Eur J Med Chem. 2021 Feb 15;212:113139. doi: 10.1016/j.ejmech.2020.113139. Epub 2020 Dec 29. Eur J Med Chem. 2021. PMID: 33422979 Review.
-
Structure-function relationships and flexible tetramer assembly in pyruvate decarboxylase revealed by analysis of crystal structures.Biochim Biophys Acta. 1998 Jun 29;1385(2):253-70. doi: 10.1016/s0167-4838(98)00073-9. Biochim Biophys Acta. 1998. PMID: 9655915 Review.
References
-
- WHO, Global Tuberculosis report, 2021
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

