Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model
- PMID: 37251648
- PMCID: PMC10213275
- DOI: 10.3389/fnmol.2023.1128545
Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model
Abstract
Objective: Disruption of the blood-spinal cord barrier (BSCB) with subsequent edema formation and further neuroinflammation contributes to aggravation of spinal cord injury (SCI). We aimed to observe the effect of antagonizing the binding of the neuropeptide Substance-P (SP) to its neurokinin-1 (NK1) receptor in a rodent SCI model.
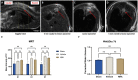
Methods: Female Wistar rats were subjected to a T9 laminectomy with or without (Sham) a T9 clip-contusion/compression SCI, followed by the implantation of an osmotic pump for the continuous, seven-day-long infusion of a NK1 receptor antagonist (NRA) or saline (vehicle) into the intrathecal space. The animals were assessed via MRI, and behavioral tests were performed during the experiment. 7 days after SCI, wet & dry weight and immunohistological analyses were conducted.
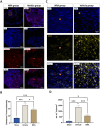
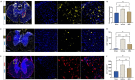
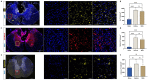
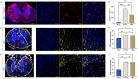
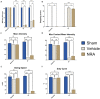
Results: Substance-P inhibition via NRA showed limited effects on reducing edema. However, the invasion of T-lymphocytes and the number of apoptotic cells were significantly reduced with the NRA treatment. Moreover, a trend of reduced fibrinogen leakage, endothelial and microglial activation, CS-GAG deposition, and astrogliosis was found. Nevertheless, only insignificant general locomotion recovery could be observed in the BBB open field score and the Gridwalk test. In contrast, the CatWalk gait analysis showed an early onset of recovery in several parameters.
Conclusion: Intrathecal administration of NRA might reinforce the integrity of the BSCB in the acute phase after SCI, potentially attenuating aspects of neurogenic inflammation, reducing edema formation, and improving functional recovery.
Keywords: animal model; behavioral assessment; blood spinal cord barrier; inflammation; neurokinin-1 (NK1) antagonist; spinal cord injury.
Copyright © 2023 Zheng, Harms, Tail, Zhang, Nimmo, Skutella, Kiening, Unterberg, Zweckberger and Younsi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sonic Hedgehog modulates the inflammatory response and improves functional recovery after spinal cord injury in a thoracic contusion-compression model.Eur Spine J. 2021 Jun;30(6):1509-1520. doi: 10.1007/s00586-021-06796-2. Epub 2021 Mar 11. Eur Spine J. 2021. PMID: 33704579
-
NK1 receptor blockade is ineffective in improving outcome following a balloon compression model of spinal cord injury.PLoS One. 2014 May 23;9(5):e98364. doi: 10.1371/journal.pone.0098364. eCollection 2014. PLoS One. 2014. PMID: 24859234 Free PMC article.
-
The effect of an NK1 receptor antagonist on blood spinal cord barrier permeability following balloon compression-induced spinal cord injury.Acta Neurochir Suppl. 2013;118:303-6. doi: 10.1007/978-3-7091-1434-6_59. Acta Neurochir Suppl. 2013. PMID: 23564154
-
Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury.Int J Mol Sci. 2021 Dec 3;22(23):13106. doi: 10.3390/ijms222313106. Int J Mol Sci. 2021. PMID: 34884911 Free PMC article.
-
Sonic Hedgehog reduces inflammatory response, decreases blood-spinal cord barrier permeability, and improves locomotor function recovery in an acute spinal cord injury rat model.J Inflamm (Lond). 2024 Sep 3;21(1):34. doi: 10.1186/s12950-024-00404-y. J Inflamm (Lond). 2024. PMID: 39227870 Free PMC article.
Cited by
-
Differential Influences of Endogenous and Exogenous Sensory Neuropeptides on the ATP Metabolism by Soluble Ectonucleotidases in the Murine Bladder Lamina Propria.Int J Mol Sci. 2023 Oct 27;24(21):15650. doi: 10.3390/ijms242115650. Int J Mol Sci. 2023. PMID: 37958631 Free PMC article.
References
-
- Badhiwala J. H., Wilson J. R., Witiw C. D., Harrop J. S., Vaccaro A. R., Aarabi B., et al. . (2021). The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet. Neurol. 20, 117–126. doi: 10.1016/S1474-4422(20)30406-3, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

