The internal dose makes the poison: higher internalization of polystyrene particles induce increased perturbation of macrophages
- PMID: 37251378
- PMCID: PMC10213243
- DOI: 10.3389/fimmu.2023.1092743
The internal dose makes the poison: higher internalization of polystyrene particles induce increased perturbation of macrophages
Abstract
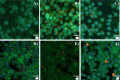
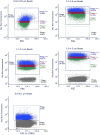
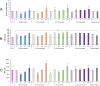
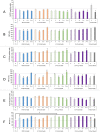
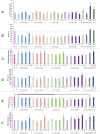
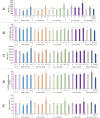
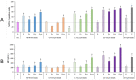
Plastics are emerging pollutants of great concern. Macroplastics released in the environment degrade into microplastics and nanoplastics. Because of their small size, these micro and nano plastic particles can enter the food chain and contaminate humans with still unknown biological effects. Plastics being particulate pollutants, they are handled in the human body by scavenger cells such as macrophages, which are important players in the innate immune system. Using polystyrene as a model of micro and nanoplastics, with size ranging from under 100 nm to 6 microns, we have showed that although non-toxic, polystyrene nano and microbeads alter the normal functioning of macrophages in a size and dose-dependent manner. Alterations in the oxidative stress, lysosomal and mitochondrial functions were detected, as well as changes in the expression of various surface markers involved in the immune response such as CD11a/b, CD18, CD86, PD-L1, or CD204. For each beads size tested, the alterations were more pronounced for the cell subpopulation that had internalized the highest number of beads. Across beads sizes, the alterations were more pronounced for beads in the supra-micron range than for beads in the sub-micron range. Overall, this means that internalization of high doses of polystyrene favors the emergence of subpopulations of macrophages with an altered phenotype, which may not only be less efficient in their functions but also alter the fine balance of the innate immune system.
Keywords: integrins; macrophages; microplastics; mitochondria; oxidative stress; polystyrene.
Copyright © 2023 Collin-Faure, Vitipon, Torres, Tanyeres, Dalzon and Rabilloud.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
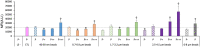
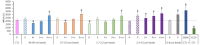
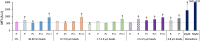
Similar articles
-
Cellular response of THP-1 macrophages to polystyrene microplastics exposure.Toxicology. 2023 Jan 1;483:153385. doi: 10.1016/j.tox.2022.153385. Epub 2022 Dec 1. Toxicology. 2023. PMID: 36464069
-
Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish.Microb Pathog. 2022 Feb;163:105387. doi: 10.1016/j.micpath.2021.105387. Epub 2022 Jan 4. Microb Pathog. 2022. PMID: 34990781
-
Cellular internalization and release of polystyrene microplastics and nanoplastics.Sci Total Environ. 2021 Jul 20;779:146523. doi: 10.1016/j.scitotenv.2021.146523. Epub 2021 Mar 18. Sci Total Environ. 2021. PMID: 34030247
-
Recent progress and future directions of the research on nanoplastic-induced neurotoxicity.Neural Regen Res. 2024 Feb;19(2):331-335. doi: 10.4103/1673-5374.379016. Neural Regen Res. 2024. PMID: 37488886 Free PMC article. Review.
-
Hazard of polystyrene micro-and nanospheres to selected aquatic and terrestrial organisms.Sci Total Environ. 2022 Dec 20;853:158560. doi: 10.1016/j.scitotenv.2022.158560. Epub 2022 Sep 7. Sci Total Environ. 2022. PMID: 36087672 Review.
Cited by
-
Microplastics: an often-overlooked issue in the transition from chronic inflammation to cancer.J Transl Med. 2024 Oct 22;22(1):959. doi: 10.1186/s12967-024-05731-5. J Transl Med. 2024. PMID: 39438955 Free PMC article. Review.
-
The in vitro gastrointestinal digestion-associated protein corona of polystyrene nano- and microplastics increases their uptake by human THP-1-derived macrophages.Part Fibre Toxicol. 2024 Feb 4;21(1):4. doi: 10.1186/s12989-024-00563-z. Part Fibre Toxicol. 2024. PMID: 38311718 Free PMC article.
-
Characterization of Nanoprecipitated PET Nanoplastics by 1H NMR and Impact of Residual Ionic Surfactant on Viability of Human Primary Mononuclear Cells and Hemolysis of Erythrocytes.Polymers (Basel). 2023 Dec 13;15(24):4703. doi: 10.3390/polym15244703. Polymers (Basel). 2023. PMID: 38139955 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

