Persistence of auditory modulation of wind-induced escape behavior in crickets
- PMID: 37250114
- PMCID: PMC10214467
- DOI: 10.3389/fphys.2023.1153913
Persistence of auditory modulation of wind-induced escape behavior in crickets
Abstract
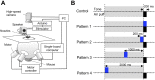
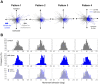
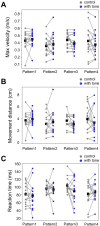
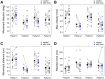
Animals, including insects, change their innate escape behavior triggered by a specific threat stimulus depending on the environmental context to survive adaptively the predators' attack. This indicates that additional inputs from sensory organs of different modalities indicating surrounding conditions could affect the neuronal circuit responsible for the escape behavior. Field crickets, Gryllus bimaculatus, exhibit an oriented running or jumping escape in response to short air puff detected by the abdominal mechanosensory organ called cerci. Crickets also receive a high-frequency acoustic stimulus by their tympanal organs on their frontal legs, which suggests approaching bats as a predator. We have reported that the crickets modulate their wind-elicited escape running in the moving direction when they are exposed to an acoustic stimulus preceded by the air puff. However, it remains unclear how long the effects of auditory inputs indicating surrounding contexts last after the sound is terminated. In this study, we applied a short pulse (200 ms) of 15-kHz pure tone to the crickets in various intervals before the air-puff stimulus. The sound given 200 or 1000 ms before the air puff biased the wind-elicited escape running backward, like the previous studies using the longer and overlapped sound. But the sounds that started 2000 ms before and simultaneously with the air puff had little effect. In addition, the jumping probability was higher only when the delay of air puff to the sound was 1000 ms. These results suggest that the cricket could retain the auditory memory for at least one second and alter the motion choice and direction of the wind-elicited escape behavior.
Keywords: behavioral choice; contextual memory; cricket (Gryllus bimaculatus); crossmodal interactions; escape behavior; multisensory integration.
Copyright © 2023 Lu, Fukutomi, Shidara and Ogawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Auditory modulation of wind-elicited walking behavior in the cricket Gryllus bimaculatus.J Exp Biol. 2015 Dec;218(Pt 24):3968-77. doi: 10.1242/jeb.128751. Epub 2015 Oct 30. J Exp Biol. 2015. PMID: 26519512
-
Behavioral analyses of wind-evoked escape of the cricket, Gryllodes sigillatus.Zoolog Sci. 2006 Apr;23(4):359-64. doi: 10.2108/zsj.23.359. Zoolog Sci. 2006. PMID: 16702769
-
Crickets alter wind-elicited escape strategies depending on acoustic context.Sci Rep. 2017 Nov 9;7(1):15158. doi: 10.1038/s41598-017-15276-x. Sci Rep. 2017. PMID: 29123249 Free PMC article.
-
Spatial perception mediated by insect antennal mechanosensory system.J Exp Biol. 2022 Feb 15;225(4):jeb243276. doi: 10.1242/jeb.243276. Epub 2022 Feb 24. J Exp Biol. 2022. PMID: 35072207 Free PMC article.
-
The neuroethology of acoustic startle and escape in flying insects.J Exp Biol. 1989 Sep;146:287-306. doi: 10.1242/jeb.146.1.287. J Exp Biol. 1989. PMID: 2689567 Review.
References
-
- Caro-Martín C., Leal-Campanario R., Sánchez-Campusano R., Delgado-García J. M., Gruart A. (2015). A variable oscillator underlies the measurement of time intervals in the rostral medial prefrontal cortex during classical eyeblink conditioning in rabbits. J. Neurosci. 35, 14809–14821. 10.1523/JNEUROSCI.2285-15.2015 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

