The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial
- PMID: 37245517
- PMCID: PMC10401723
- DOI: 10.1016/S0140-6736(23)00806-1
The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial
Erratum in
-
Department of Error.Lancet. 2023 Jul 15;402(10397):184. doi: 10.1016/S0140-6736(23)01420-4. Lancet. 2023. PMID: 37453751 Free PMC article. No abstract available.
Abstract
Background: Early control of elevated blood pressure is the most promising treatment for acute intracerebral haemorrhage. We aimed to establish whether implementing a goal-directed care bundle incorporating protocols for early intensive blood pressure lowering and management algorithms for hyperglycaemia, pyrexia, and abnormal anticoagulation, implemented in a hospital setting, could improve outcomes for patients with acute spontaneous intracerebral haemorrhage.
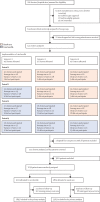
Methods: We performed a pragmatic, international, multicentre, blinded endpoint, stepped wedge cluster randomised controlled trial at hospitals in nine low-income and middle-income countries (Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, and Viet Nam) and one high-income country (Chile). Hospitals were eligible if they had no or inconsistent relevant, disease-specific protocols, and were willing to implement the care bundle to consecutive patients (aged ≥18 years) with imaging-confirmed spontaneous intracerebral haemorrhage presenting within 6 h of the onset of symptoms, had a local champion, and could provide the required study data. Hospitals were centrally randomly allocated using permuted blocks to three sequences of implementation, stratified by country and the projected number of patients to be recruited over the 12 months of the study period. These sequences had four periods that dictated the order in which the hospitals were to switch from the control usual care procedure to the intervention implementation of the care bundle procedure to different clusters of patients in a stepped manner. To avoid contamination, details of the intervention, sequence, and allocation periods were concealed from sites until they had completed the usual care control periods. The care bundle protocol included the early intensive lowering of systolic blood pressure (target <140 mm Hg), strict glucose control (target 6·1-7·8 mmol/L in those without diabetes and 7·8-10·0 mmol/L in those with diabetes), antipyrexia treatment (target body temperature ≤37·5°C), and rapid reversal of warfarin-related anticoagulation (target international normalised ratio <1·5) within 1 h of treatment, in patients where these variables were abnormal. Analyses were performed according to a modified intention-to-treat population with available outcome data (ie, excluding sites that withdrew during the study). The primary outcome was functional recovery, measured with the modified Rankin scale (mRS; range 0 [no symptoms] to 6 [death]) at 6 months by masked research staff, analysed using proportional ordinal logistic regression to assess the distribution in scores on the mRS, with adjustments for cluster (hospital site), group assignment of cluster per period, and time (6-month periods from Dec 12, 2017). This trial is registered at Clinicaltrials.gov (NCT03209258) and the Chinese Clinical Trial Registry (ChiCTR-IOC-17011787) and is completed.
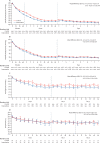
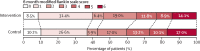
Findings: Between May 27, 2017, and July 8, 2021, 206 hospitals were assessed for eligibility, of which 144 hospitals in ten countries agreed to join and were randomly assigned in the trial, but 22 hospitals withdrew before starting to enrol patients and another hospital was withdrawn and their data on enrolled patients was deleted because regulatory approval was not obtained. Between Dec 12, 2017, and Dec 31, 2021, 10 857 patients were screened but 3821 were excluded. Overall, the modified intention-to-treat population included 7036 patients enrolled at 121 hospitals, with 3221 assigned to the care bundle group and 3815 to the usual care group, with primary outcome data available in 2892 patients in the care bundle group and 3363 patients in the usual care group. The likelihood of a poor functional outcome was lower in the care bundle group (common odds ratio 0·86; 95% CI 0·76-0·97; p=0·015). The favourable shift in mRS scores in the care bundle group was generally consistent across a range of sensitivity analyses that included additional adjustments for country and patient variables (0·84; 0·73-0·97; p=0·017), and with different approaches to the use of multiple imputations for missing data. Patients in the care bundle group had fewer serious adverse events than those in the usual care group (16·0% vs 20·1%; p=0·0098).
Interpretation: Implementation of a care bundle protocol for intensive blood pressure lowering and other management algorithms for physiological control within several hours of the onset of symptoms resulted in improved functional outcome for patients with acute intracerebral haemorrhage. Hospitals should incorporate this approach into clinical practice as part of active management for this serious condition.
Funding: Joint Global Health Trials scheme from the Department of Health and Social Care, the Foreign, Commonwealth & Development Office, and the Medical Research Council and Wellcome Trust; West China Hospital; the National Health and Medical Research Council of Australia; Sichuan Credit Pharmaceutic and Takeda China.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests LS reports funding from the Medical Research Council of the UK, Sichuan Credit Pharmaceutic, and Takeda China; and speaker fees from Takeda China. CSA has received grants from the National Health and Medical Research Council and Medical Research Futures Fund of Australia, the Medical Research Council of the UK, Penumbra, and Takeda China; is also the chair of the data and safety monitoring boards for several trials; is a board member of WHO; and is the Editor-in-Chief of Cerebrovascular Disease. CY has received funding from West China Hospital. All other authors declare no competing interests.
Figures
Comment in
-
Acute spontaneous intracerebral haemorrhage: does a care bundle approach work?Lancet. 2023 Jul 1;402(10395):2-3. doi: 10.1016/S0140-6736(23)00911-X. Epub 2023 May 25. Lancet. 2023. PMID: 37245518 No abstract available.
Similar articles
-
INTEnsive care bundle with blood pressure reduction in acute cerebral hemorrhage trial (INTERACT3): study protocol for a pragmatic stepped-wedge cluster-randomized controlled trial.Trials. 2021 Dec 20;22(1):943. doi: 10.1186/s13063-021-05881-7. Trials. 2021. PMID: 34930428 Free PMC article.
-
Statistical Analysis Plan for the INTEnsive Care Bundle with Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial: A Stepped-Wedge Cluster Randomized Controlled Trial.Cerebrovasc Dis. 2023;52(3):251-254. doi: 10.1159/000526384. Epub 2022 Sep 5. Cerebrovasc Dis. 2023. PMID: 36063792 Free PMC article. Clinical Trial.
-
Implementing a Goal-Directed Care Bundle after Acute Intracerebral Haemorrhage: Process Evaluation for the Third INTEnsive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial Study in China.Cerebrovasc Dis. 2022;51(3):373-383. doi: 10.1159/000520669. Epub 2021 Dec 20. Cerebrovasc Dis. 2022. PMID: 34929690 Clinical Trial.
-
The Prevention of Delirium system of care for older patients admitted to hospital for emergency care: the POD research programme including feasibility RCT.Southampton (UK): NIHR Journals Library; 2021 Mar. Southampton (UK): NIHR Journals Library; 2021 Mar. PMID: 33819001 Free Books & Documents. Review.
-
Blood Pressure Management in Intracerebral Haemorrhage: when, how much, and for how long?Curr Neurol Neurosci Rep. 2024 Jul;24(7):181-189. doi: 10.1007/s11910-024-01341-2. Epub 2024 May 23. Curr Neurol Neurosci Rep. 2024. PMID: 38780706 Free PMC article. Review.
Cited by
-
Continuous Blood Pressure Indices During the First 72 Hours and Functional Outcome in Patients with Spontaneous Intracerebral Hemorrhage.Neurocrit Care. 2024 Oct 25. doi: 10.1007/s12028-024-02146-4. Online ahead of print. Neurocrit Care. 2024. PMID: 39455525
-
Development of a risk predication model for critical care needs in patients with intracerebral hemorrhage: a retrospective cohort.BMC Nurs. 2024 Oct 19;23(1):770. doi: 10.1186/s12912-024-02319-8. BMC Nurs. 2024. PMID: 39427213 Free PMC article.
-
Postoperative fever and clinical outcomes after endoscopic surgery for spontaneous intracerebral hemorrhage: a retrospective database study.BMC Neurol. 2024 Oct 15;24(1):392. doi: 10.1186/s12883-024-03898-4. BMC Neurol. 2024. PMID: 39407147 Free PMC article.
-
Predicting Intracerebral Hemorrhage Expansion with Inflammation Indices, Non-Contrast Computed Tomography Signs and Computed Tomography Angiography Spot Sign.Neuropsychiatr Dis Treat. 2024 Oct 3;20:1879-1887. doi: 10.2147/NDT.S475550. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 39376667 Free PMC article.
-
Blood Pressure Management in Acute Ischemic Stroke With Concurrent Intracranial Neoplasm and Intratumoral Hemorrhage.Cureus. 2024 Aug 28;16(8):e68045. doi: 10.7759/cureus.68045. eCollection 2024 Aug. Cureus. 2024. PMID: 39347201 Free PMC article.
References
-
- Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392:1257–1268. - PubMed
-
- Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53:e282–e361. - PubMed
-
- Stroke Foundation Living Clinical Guidelines for Stroke Management. Chapter 3 Acute medical and surgical management. https://informme.org.au/en/Guidelines/Living-Clinical-Guidelines-for-Str...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

