Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization
- PMID: 37244989
- PMCID: PMC10221750
- DOI: 10.1186/s12977-023-00624-9
Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization
Abstract
Background: Neutralizing antibodies (NAbs) protect against HIV-1 acquisition in animal models and show promise in treatment of infection. They act by binding to the viral envelope glycoprotein (Env), thereby blocking its receptor interactions and fusogenic function. The potency of neutralization is largely determined by affinity. Less well explained is the persistent fraction, the plateau of remaining infectivity at the highest antibody concentrations.
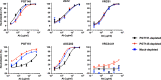
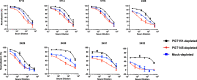
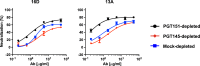
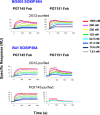
Results: We observed different persistent fractions for neutralization of pseudovirus derived from two Tier-2 isolates of HIV-1, BG505 (Clade A) and B41 (Clade B): it was pronounced for B41 but not BG505 neutralization by NAb PGT151, directed to the interface between the outer and transmembrane subunits of Env, and negligible for either virus by NAb PGT145 to an apical epitope. Autologous neutralization by poly- and monoclonal NAbs from rabbits immunized with soluble native-like B41 trimer also left substantial persistent fractions. These NAbs largely target a cluster of epitopes lining a hole in the dense glycan shield of Env around residue 289. We partially depleted B41-virion populations by incubating them with PGT145- or PGT151-conjugated beads. Each depletion reduced the sensitivity to the depleting NAb and enhanced it to the other. Autologous neutralization by the rabbit NAbs was decreased for PGT145-depleted and enhanced for PGT151-depleted B41 pseudovirus. Those changes in sensitivity encompassed both potency and the persistent fraction. We then compared soluble native-like BG505 and B41 Env trimers affinity-purified by each of three NAbs: 2G12, PGT145, or PGT151. Surface plasmon resonance showed differences among the fractions in antigenicity, including kinetics and stoichiometry, congruently with the differential neutralization. The large persistent fraction after PGT151 neutralization of B41 was attributable to low stoichiometry, which we explained structurally by clashes that the conformational plasticity of B41 Env causes.
Conclusion: Distinct antigenic forms even of clonal HIV-1 Env, detectable among soluble native-like trimer molecules, are distributed over virions and may profoundly mold neutralization of certain isolates by certain NAbs. Affinity purifications with some antibodies may yield immunogens that preferentially expose epitopes for broadly active NAbs, shielding less cross-reactive ones. NAbs reactive with multiple conformers will together reduce the persistent fraction after passive and active immunization.
Keywords: Antigenic heterogeneity; Binding kinetics; Broadly active neutralizing antibodies (bNAbs); Efficacy; HIV-1 neutralization; Persistent fraction; Stoichiometry.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Update of
-
Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.Res Sq [Preprint]. 2023 Feb 24:rs.3.rs-2613503. doi: 10.21203/rs.3.rs-2613503/v1. Res Sq. 2023. Update in: Retrovirology. 2023 May 27;20(1):9. doi: 10.1186/s12977-023-00624-9. PMID: 36865101 Free PMC article. Updated. Preprint.
Similar articles
-
Glycan heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.PLoS Pathog. 2023 Oct 30;19(10):e1011601. doi: 10.1371/journal.ppat.1011601. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37903160 Free PMC article.
-
Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.Res Sq [Preprint]. 2023 Feb 24:rs.3.rs-2613503. doi: 10.21203/rs.3.rs-2613503/v1. Res Sq. 2023. Update in: Retrovirology. 2023 May 27;20(1):9. doi: 10.1186/s12977-023-00624-9. PMID: 36865101 Free PMC article. Updated. Preprint.
-
Closing and Opening Holes in the Glycan Shield of HIV-1 Envelope Glycoprotein SOSIP Trimers Can Redirect the Neutralizing Antibody Response to the Newly Unmasked Epitopes.J Virol. 2019 Feb 5;93(4):e01656-18. doi: 10.1128/JVI.01656-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487280 Free PMC article.
-
The HIV-1 envelope glycoprotein structure: nailing down a moving target.Immunol Rev. 2017 Jan;275(1):21-32. doi: 10.1111/imr.12507. Immunol Rev. 2017. PMID: 28133813 Free PMC article. Review.
-
Passive immunization as tool to identify protective HIV-1 Env epitopes.Curr HIV Res. 2007 Nov;5(6):642-55. doi: 10.2174/157016207782418506. Curr HIV Res. 2007. PMID: 18045119 Review.
Cited by
-
Glycan heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.PLoS Pathog. 2023 Oct 30;19(10):e1011601. doi: 10.1371/journal.ppat.1011601. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37903160 Free PMC article.
-
Impact of glycan depletion, glycan debranching and increased glycan charge on HIV-1 neutralization sensitivity and immunogenicity.Glycobiology. 2024 Sep 30;34(11):cwae063. doi: 10.1093/glycob/cwae063. Glycobiology. 2024. PMID: 39115361
References
-
- Burton DR, Saphire EO, Parren PW. A model for neutralization of viruses based on antibody coating of the virion surface. Curr Top Microbiol Immunol. 2001;260:109–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

