ROS-Induced Mitochondrial Dysfunction in CD4 T Cells from ART-Controlled People Living with HIV
- PMID: 37243148
- PMCID: PMC10224005
- DOI: 10.3390/v15051061
ROS-Induced Mitochondrial Dysfunction in CD4 T Cells from ART-Controlled People Living with HIV
Abstract
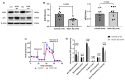
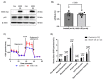
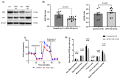
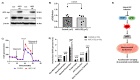
We have previously demonstrated mitochondrial dysfunction in aging CD4 T cells from antiretroviral therapy (ART)-controlled people living with HIV (PLWH). However, the underlying mechanisms by which CD4 T cells develop mitochondrial dysfunction in PLWH remain unclear. In this study, we sought to elucidate the mechanism(s) of CD4 T cell mitochondrial compromise in ART-controlled PLWH. We first assessed the levels of reactive oxygen species (ROS), and we observed significantly increased cellular and mitochondrial ROS levels in CD4 T cells from PLWH compared to healthy subjects (HS). Furthermore, we observed a significant reduction in the levels of proteins responsible for antioxidant defense (superoxide dismutase 1, SOD1) and ROS-mediated DNA damage repair (apurinic/apyrimidinic endonuclease 1, APE1) in CD4 T cells from PLWH. Importantly, CRISPR/Cas9-mediated knockdown of SOD1 or APE1 in CD4 T cells from HS confirmed their roles in maintaining normal mitochondrial respiration via a p53-mediated pathway. Reconstitution of SOD1 or APE1 in CD4 T cells from PLWH successfully rescued mitochondrial function as evidenced by Seahorse analysis. These results indicate that ROS induces mitochondrial dysfunction, leading to premature T cell aging via dysregulation of SOD1 and APE1 during latent HIV infection.
Keywords: PLWH; T cell aging; mitochondrial dysfunction; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Circulating GDF-15: a biomarker for metabolic dysregulation and aging in people living with HIV.Front Aging. 2024 Jun 4;5:1414866. doi: 10.3389/fragi.2024.1414866. eCollection 2024. Front Aging. 2024. PMID: 38895099 Free PMC article.
-
Mitochondrial topoisomerase 1 inhibition induces topological DNA damage and T cell dysfunction in patients with chronic viral infection.Front Cell Infect Microbiol. 2022 Nov 3;12:1026293. doi: 10.3389/fcimb.2022.1026293. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36405960 Free PMC article.
-
Long Non-coding RNA GAS5 Regulates T Cell Functions via miR21-Mediated Signaling in People Living With HIV.Front Immunol. 2021 Mar 12;12:601298. doi: 10.3389/fimmu.2021.601298. eCollection 2021. Front Immunol. 2021. PMID: 33776993 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
Accelerated Neuroimmune Dysfunction in Aged HIV-1-Infected Humanized Mice.Pharmaceuticals (Basel). 2024 Jan 23;17(2):149. doi: 10.3390/ph17020149. Pharmaceuticals (Basel). 2024. PMID: 38399364 Free PMC article.
-
The master antioxidant defense is activated during EBV latent infection.J Virol. 2023 Nov 30;97(11):e0095323. doi: 10.1128/jvi.00953-23. Epub 2023 Oct 25. J Virol. 2023. PMID: 37877721 Free PMC article.
-
Enhanced ROS Production and Mitochondrial Metabolic Shifts in CD4+ T Cells of an Autoimmune Uveitis Model.Int J Mol Sci. 2024 Oct 26;25(21):11513. doi: 10.3390/ijms252111513. Int J Mol Sci. 2024. PMID: 39519064 Free PMC article.
-
HIV-1-related factors interact with p53 to influence cellular processes.AIDS Res Ther. 2023 Sep 10;20(1):66. doi: 10.1186/s12981-023-00563-7. AIDS Res Ther. 2023. PMID: 37691100 Free PMC article. Review.
-
CRISPR-Cas9 applications in T cells and adoptive T cell therapies.Cell Mol Biol Lett. 2024 Apr 12;29(1):52. doi: 10.1186/s11658-024-00561-1. Cell Mol Biol Lett. 2024. PMID: 38609863 Free PMC article. Review.
References
-
- Jimnez V.C., Wit F.W.N.M., Joerink M., Maurer I., Harskamp A.M., Schouten J., Prins M., Van Leeuwen E.M.M., Booiman T., Deeks S.G., et al. T-Cell Activation Independently Associates with Immune Senescence in HIV-Infected Recipients of Long-Term Antiretroviral Treatment. J. Infect. Dis. 2016;214:216–225. doi: 10.1093/infdis/jiw146. - DOI - PMC - PubMed
-
- Pathai S., Lawn S.D., Gilbert C.E., McGuinness D., McGlynn L., Weiss H.A., Port J., Christ T., Barclay K., Wood R., et al. Accelerated Biological Ageing in HIV-Infected Individuals in South Africa: A Case–Control Study. AIDS. 2013;27:2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. - DOI - PMC - PubMed
-
- Zanet D.A.L., Thorne A., Singer J., Maan E.J., Sattha B., Le Campion A., Soudeyns H., Pick N., Murray M., Money D.M., et al. Association between Short Leukocyte Telomere Length and HIV Infection in a Cohort Study: No Evidence of a Relationship with Antiretroviral Therapy. Clin. Infect. Dis. 2014;58:1322–1332. doi: 10.1093/cid/ciu051. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

