The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines
- PMID: 37242529
- PMCID: PMC10221675
- DOI: 10.3390/ph16050746
The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines
Abstract
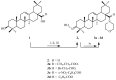
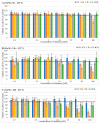
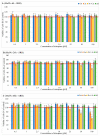
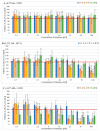
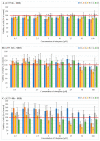
This study aimed to synthesize four new semisynthetic derivatives of natural oleanolic acid (OA) and, based on an analysis of their cytotoxic and anti-proliferative effects against human MeWo and A375 melanoma cell lines, select those with anti-cancer potential. We also screened the treatment time with the concentration of all four derivatives. We synthesized oxime 2 and performed its acylation with carboxylic acids into new derivatives 3a, 3b, 3c and 3d according to the methods previously described. Colorimetric MTT and SRB assays were used to measure the anti-proliferative and cytotoxic activity of OA and its derivatives 3a, 3b, 3c and 3d against melanoma cells. Selected concentrations of OA, the derivatives, and different time periods of incubation were used in the study. The data were analyzed statistically. The present results revealed the possible anti-proliferative and cytotoxic potential of two selected OA derivatives 3a and 3b, on A375 and MeWo melanoma cells, especially at concentrations of 50 μM and 100 μM at 48 h of incubation (p < 0.05). Further studies will be necessary to analyze the proapoptotic and anti-cancer activities of 3a and 3b against skin and other cancer cells. The bromoacetoxyimine derivative (3b) of OA morpholide turned out to be the most effective against the tested cancer cells.
Keywords: cytotoxicity; human melanoma; oleanolic acid derivatives; triterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis of bis-Chalcones Based on Green Chemistry Strategies and Their Cytotoxicity Toward Human MeWo and A375 Melanoma Cell Lines.Molecules. 2024 Oct 31;29(21):5171. doi: 10.3390/molecules29215171. Molecules. 2024. PMID: 39519811 Free PMC article.
-
Synthesis of Oleanolic Acid Analogues and Their Cytotoxic Effects on 3T3 Cell Line.Med Chem. 2018;14(6):617-625. doi: 10.2174/1573406414666180222094544. Med Chem. 2018. PMID: 29473521
-
Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies.Pharmaceutics. 2024 Jan 9;16(1):86. doi: 10.3390/pharmaceutics16010086. Pharmaceutics. 2024. PMID: 38258097 Free PMC article.
-
Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs.Molecules. 2021 Aug 16;26(16):4957. doi: 10.3390/molecules26164957. Molecules. 2021. PMID: 34443544 Free PMC article. Review.
-
A Review on Recent Developments in the Anticancer Potential of Oleanolic Acid and its Analogs (2017-2020).Mini Rev Med Chem. 2022;22(4):600-616. doi: 10.2174/1389557521666210810153627. Mini Rev Med Chem. 2022. PMID: 35135459 Review.
Cited by
-
Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues.Molecules. 2024 Jul 12;29(14):3291. doi: 10.3390/molecules29143291. Molecules. 2024. PMID: 39064870 Free PMC article. Review.
-
Semisynthetic phytochemicals in cancer treatment: a medicinal chemistry perspective.RSC Med Chem. 2024 Aug 7;15(10):3345-3370. doi: 10.1039/d4md00317a. eCollection 2024 Oct 17. RSC Med Chem. 2024. PMID: 39430100 Review.
-
Selectivity Screening and Structure-Cytotoxic Activity Observations of Selected Oleanolic Acid (OA)-Type Saponins from the Amaranthaceae Family on a Wiade Panel of Human Cancer Cell Lines.Molecules. 2024 Aug 10;29(16):3794. doi: 10.3390/molecules29163794. Molecules. 2024. PMID: 39202875 Free PMC article.
-
Synthesis of bis-Chalcones Based on Green Chemistry Strategies and Their Cytotoxicity Toward Human MeWo and A375 Melanoma Cell Lines.Molecules. 2024 Oct 31;29(21):5171. doi: 10.3390/molecules29215171. Molecules. 2024. PMID: 39519811 Free PMC article.
-
An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates.Antioxidants (Basel). 2024 Aug 6;13(8):952. doi: 10.3390/antiox13080952. Antioxidants (Basel). 2024. PMID: 39199198 Free PMC article. Review.
References
-
- Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Basset-Seguin N., Bastholt L., Bataille V., del Marmol V., Dréno B., et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer. 2022;170:236–255. doi: 10.1016/j.ejca.2022.03.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

