Galactic Cosmic Irradiation Alters Acute and Delayed Species-Typical Behavior in Male and Female Mice
- PMID: 37240858
- PMCID: PMC10223209
- DOI: 10.3390/life13051214
Galactic Cosmic Irradiation Alters Acute and Delayed Species-Typical Behavior in Male and Female Mice
Abstract
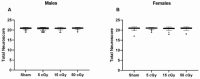
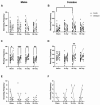
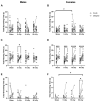
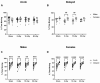
Exposure to space galactic cosmic radiation is a principal consideration for deep space missions. While the effects of space irradiation on the nervous system are not fully known, studies in animal models have shown that exposure to ionizing radiation can cause neuronal damage and lead to downstream cognitive and behavioral deficits. Cognitive health implications put humans and missions at risk, and with the upcoming Artemis missions in which female crew will play a major role, advance critical analysis of the neurological and performance responses of male and female rodents to space radiation is vital. Here, we tested the hypothesis that simulated Galactic Cosmic Radiation (GCRSim) exposure disrupts species-typical behavior in mice, including burrowing, rearing, grooming, and nest-building that depend upon hippocampal and medial prefrontal cortex circuitry. Behavior comprises a remarkably well-integrated representation of the biology of the whole animal that informs overall neural and physiological status, revealing functional impairment. We conducted a systematic dose-response analysis of mature (6-month-old) male and female mice exposed to either 5, 15, or 50 cGy 5-ion GCRSim (H, Si, He, O, Fe) at the NASA Space Radiation Laboratory (NSRL). Behavioral performance was evaluated at 72 h (acute) and 91-days (delayed) postradiation exposure. Specifically, species-typical behavior patterns comprising burrowing, rearing, and grooming as well as nest building were analyzed. A Neuroscore test battery (spontaneous activity, proprioception, vibrissae touch, limb symmetry, lateral turning, forelimb outstretching, and climbing) was performed at the acute timepoint to investigate early sensorimotor deficits postirradiation exposure. Nest construction, a measure of neurological and organizational function in rodents, was evaluated using a five-stage Likert scale 'Deacon' score that ranged from 1 (a low score where the Nestlet is untouched) to 5 (a high score where the Nestlet is completely shredded and shaped into a nest). Differential acute responses were observed in females relative to males with respect to species-typical behavior following 15 cGy exposure while delayed responses were observed in female grooming following 50 cGy exposure. Significant sex differences were observed at both timepoints in nest building. No deficits in sensorimotor behavior were observed via the Neuroscore. This study revealed subtle, sexually dimorphic GCRSim exposure effects on mouse behavior. Our analysis provides a clearer understanding of GCR dose effects on species typical, sensorimotor and organizational behaviors at acute and delayed timeframes postirradiation, thereby setting the stage for the identification of underlying cellular and molecular events.
Keywords: GCR radiation exposure; cognition; rodent behavior; sex differences.
Conflict of interest statement
The authors declare no conflict of interest. Research was sponsored by the National Aeronautics and Space Administration (NASA) through a contract with Oak Ridge Associated Universities. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Aeronautics and Space Administration (NASA) or the U.S. Government. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
NASA's first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research.PLoS Biol. 2020 May 19;18(5):e3000669. doi: 10.1371/journal.pbio.3000669. eCollection 2020 May. PLoS Biol. 2020. PMID: 32428004 Free PMC article.
-
Simulated galactic cosmic radiation-induced cancer progression in mice.Life Sci Space Res (Amst). 2024 May;41:43-51. doi: 10.1016/j.lssr.2024.01.008. Epub 2024 Feb 1. Life Sci Space Res (Amst). 2024. PMID: 38670651
-
Evaluating the effects of low-dose simulated galactic cosmic rays on murine hippocampal-dependent cognitive performance.Front Neurosci. 2022 Dec 6;16:908632. doi: 10.3389/fnins.2022.908632. eCollection 2022. Front Neurosci. 2022. PMID: 36561122 Free PMC article.
-
Galactic cosmic ray simulation at the NASA space radiation laboratory - Progress, challenges and recommendations on mixed-field effects.Life Sci Space Res (Amst). 2023 Feb;36:90-104. doi: 10.1016/j.lssr.2022.09.001. Epub 2022 Sep 10. Life Sci Space Res (Amst). 2023. PMID: 36682835 Review.
-
Microglia: Ally and Enemy in Deep Space.Neurosci Biobehav Rev. 2021 Jul;126:509-514. doi: 10.1016/j.neubiorev.2021.03.036. Epub 2021 Apr 16. Neurosci Biobehav Rev. 2021. PMID: 33862064 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

