The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD
- PMID: 37240472
- PMCID: PMC10219583
- DOI: 10.3390/jcm12103366
The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD
Abstract
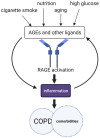
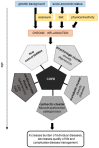
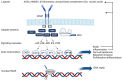
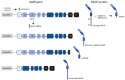
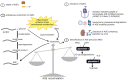
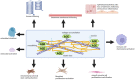
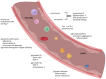
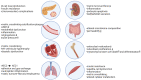
Chronic obstructive pulmonary disease (COPD) is a disease of the airways and lungs due to an enhanced inflammatory response, commonly caused by cigarette smoking. Patients with COPD are often multimorbid, as they commonly suffer from multiple chronic (inflammatory) conditions. This intensifies the burden of individual diseases, negatively affects quality of life, and complicates disease management. COPD and comorbidities share genetic and lifestyle-related risk factors and pathobiological mechanisms, including chronic inflammation and oxidative stress. The receptor for advanced glycation end products (RAGE) is an important driver of chronic inflammation. Advanced glycation end products (AGEs) are RAGE ligands that accumulate due to aging, inflammation, oxidative stress, and carbohydrate metabolism. AGEs cause further inflammation and oxidative stress through RAGE, but also through RAGE-independent mechanisms. This review describes the complexity of RAGE signaling and the causes of AGE accumulation, followed by a comprehensive overview of alterations reported on AGEs and RAGE in COPD and in important co-morbidities. Furthermore, it describes the mechanisms by which AGEs and RAGE contribute to the pathophysiology of individual disease conditions and how they execute crosstalk between organ systems. A section on therapeutic strategies that target AGEs and RAGE and could alleviate patients from multimorbid conditions using single therapeutics concludes this review.
Keywords: COPD; advanced glycation end products; aging; chronic inflammation; multimorbidity; receptor for advanced glycation end products.
Conflict of interest statement
L.E.G.W.V. received payment for lectures and advisory boards from Astra Zeneca, GSK, Boehringer, Novartis, Chiesi, Pulmonx, and Resmed, outside the scope of the current manuscript. T.N.P. received payments for an advisory board from Arrowhead Pharmaceuticals. N.L.R. declares no conflict of interest.
Figures
Similar articles
-
The AGE-RAGE Axis and RAGE Genetics in Chronic Obstructive Pulmonary Disease.Clin Rev Allergy Immunol. 2021 Apr;60(2):244-258. doi: 10.1007/s12016-020-08815-4. Epub 2020 Nov 10. Clin Rev Allergy Immunol. 2021. PMID: 33170477 Review.
-
Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease.Int J Biochem Cell Biol. 2016 Dec;81(Pt B):403-418. doi: 10.1016/j.biocel.2016.06.016. Epub 2016 Jun 29. Int J Biochem Cell Biol. 2016. PMID: 27373680 Review.
-
The receptor for advanced glycation end products (RAGE) is involved in mitochondrial function and cigarette smoke-induced oxidative stress.Free Radic Biol Med. 2023 Feb 1;195:261-269. doi: 10.1016/j.freeradbiomed.2022.12.089. Epub 2022 Dec 29. Free Radic Biol Med. 2023. PMID: 36586455
-
Implication of RAGE Polymorphic Variants in COPD Complication and Anti-COPD Therapeutic Potential of sRAGE.COPD. 2021 Dec;18(6):737-748. doi: 10.1080/15412555.2021.1984417. Epub 2021 Oct 6. COPD. 2021. PMID: 34615424 Review.
-
Receptor for advanced glycation end products: a new theraputic target for chronic obstructive pulmonary disease?Arch Med Res. 2013 Jan;44(1):75-6. doi: 10.1016/j.arcmed.2012.12.003. Epub 2013 Jan 1. Arch Med Res. 2013. PMID: 23287523
Cited by
-
Glucocorticoids in lung cancer: Navigating the balance between immunosuppression and therapeutic efficacy.Heliyon. 2024 Jun 4;10(12):e32357. doi: 10.1016/j.heliyon.2024.e32357. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022002 Free PMC article. Review.
-
High mobility group box 1 in the central nervous system: regeneration hidden beneath inflammation.Neural Regen Res. 2025 Jan 1;20(1):107-115. doi: 10.4103/NRR.NRR-D-23-01964. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 38767480 Free PMC article.
-
Infertility in Fabry's Disease: role of hypoxia and inflammation in determining testicular damage.Front Endocrinol (Lausanne). 2024 Feb 22;15:1340188. doi: 10.3389/fendo.2024.1340188. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38455658 Free PMC article.
-
MicroRNAs as promising drug delivery target to ameliorate chronic obstructive pulmonary disease using nano-carriers: a comprehensive review.Mol Cell Biochem. 2024 Sep 10. doi: 10.1007/s11010-024-05110-0. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39254870 Review.
-
Advanced Glycation End Products and Health: A Systematic Review.Ann Biomed Eng. 2024 Dec;52(12):3145-3156. doi: 10.1007/s10439-024-03499-9. Epub 2024 May 5. Ann Biomed Eng. 2024. PMID: 38705931 Review.
References
-
- Pahal P., Hashmi M.F., Sharma S. Chronic Obstructive Pulmonary Disease Compensatory Measures. StatPearls; Treasure Island, FL, USA: 2022. - PubMed
-
- dos Santos N.C., Miravitlles M., Camelier A.A., de Almeida V.D.C., Maciel R.R.B.T., Camelier F.W.R. Prevalence and Impact of Comorbidities in Individuals with Chronic Obstructive Pulmonary Disease: A Systematic Review. Tuberc. Respir. Dis. 2022;85:205–220. doi: 10.4046/trd.2021.0179. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

