Butyrophilins: Dynamic Regulators of Protective T Cell Immunity in Cancer
- PMID: 37240071
- PMCID: PMC10218201
- DOI: 10.3390/ijms24108722
Butyrophilins: Dynamic Regulators of Protective T Cell Immunity in Cancer
Abstract
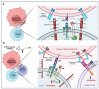
The efficacy of current immunotherapies remains limited in many solid epithelial malignancies. Recent investigations into the biology of butyrophilin (BTN) and butyrophilin-like (BTNL) molecules, however, suggest these molecules are potent immunosuppressors of antigen-specific protective T cell activity in tumor beds. BTN and BTNL molecules also associate with each other dynamically on cellular surfaces in specific contexts, which modulates their biology. At least in the case of BTN3A1, this dynamism drives the immunosuppression of αβ T cells or the activation of Vγ9Vδ2 T cells. Clearly, there is much to learn regarding the biology of BTN and BTNL molecules in the context of cancer, where they may represent intriguing immunotherapeutic targets that could potentially synergize with the current class of immune modulators in cancer. Here, we discuss our current understanding of BTN and BTNL biology, with a particular focus on BTN3A1, and potential therapeutic implications for cancer.
Keywords: butyrophilins; immune oncology; immune suppression; immunotherapy; γδ T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology.Proc Natl Acad Sci U S A. 2018 Jan 30;115(5):1039-1044. doi: 10.1073/pnas.1701237115. Epub 2018 Jan 16. Proc Natl Acad Sci U S A. 2018. PMID: 29339503 Free PMC article.
-
Butyrophilins: γδ T Cell Receptor Ligands, Immunomodulators and More.Front Immunol. 2022 Mar 17;13:876493. doi: 10.3389/fimmu.2022.876493. eCollection 2022. Front Immunol. 2022. PMID: 35371078 Free PMC article. Review.
-
From immunomodulation to therapeutic prospects: Unveiling the biology of butyrophilins in cancer.Cell Biochem Funct. 2024 Jul;42(5):e4081. doi: 10.1002/cbf.4081. Cell Biochem Funct. 2024. PMID: 38934382 Review.
-
Butyrophilin-like 3 Directly Binds a Human Vγ4+ T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen.Immunity. 2019 Nov 19;51(5):813-825.e4. doi: 10.1016/j.immuni.2019.09.006. Epub 2019 Oct 15. Immunity. 2019. PMID: 31628053 Free PMC article.
-
The Juxtamembrane Domain of Butyrophilin BTN3A1 Controls Phosphoantigen-Mediated Activation of Human Vγ9Vδ2 T Cells.J Immunol. 2017 Jun 1;198(11):4228-4234. doi: 10.4049/jimmunol.1601910. Epub 2017 May 1. J Immunol. 2017. PMID: 28461569
Cited by
-
A Ménage à trois: NLRC5, immunity, and metabolism.Front Immunol. 2024 Jul 5;15:1426620. doi: 10.3389/fimmu.2024.1426620. eCollection 2024. Front Immunol. 2024. PMID: 39035010 Free PMC article. Review.
-
The Tumor Microenvironment and Immune Response in Breast Cancer.Int J Mol Sci. 2024 Jan 11;25(2):914. doi: 10.3390/ijms25020914. Int J Mol Sci. 2024. PMID: 38255987 Free PMC article.
References
-
- Stephen T.L., Payne K.K., Chaurio R.A., Allegrezza M.J., Zhu H., Perez-Sanz J., Perales-Puchalt A., Nguyen J.M., Vara-Ailor A.E., Eruslanov E.B., et al. SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells. Immunity. 2017;46:51–64. doi: 10.1016/j.immuni.2016.12.015. - DOI - PMC - PubMed
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

