Modulating GPCR and 14-3-3 protein interactions: Prospects for CNS drug discovery
- PMID: 37236523
- PMCID: PMC10524340
- DOI: 10.1016/j.drudis.2023.103641
Modulating GPCR and 14-3-3 protein interactions: Prospects for CNS drug discovery
Abstract
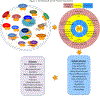
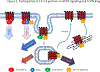
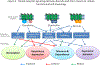
The activation of G-protein-coupled receptors (GPCRs) triggers a series of protein-protein interaction events that subsequently induce a chain of reactions, including alteration of receptor structures, phosphorylation, recruitment of associated proteins, protein trafficking and gene expression. Multiple GPCR signaling transduction pathways are evident - two well-studied pathways are the GPCR-mediated G-protein and β-arrestin pathways. Recently, ligand-induced interactions between GPCRs and 14-3-3 proteins have been demonstrated. This linking of GPCRs to 14-3-3 protein signal hubs opens up a whole new realm of signal transduction possibilities. 14-3-3 proteins play a key part in GPCR trafficking and signal transduction. GPCR-mediated 14-3-3 protein signaling can be harnessed for the study of GPCR function and therapeutics.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of interest
H.E. is an inventor of the patented LinkLight™ technology. S.K. declares no competing interests.
Figures
Similar articles
-
14-3-3 signal adaptor and scaffold proteins mediate GPCR trafficking.Sci Rep. 2019 Aug 1;9(1):11156. doi: 10.1038/s41598-019-47478-w. Sci Rep. 2019. PMID: 31371790 Free PMC article.
-
β-arrestins and G protein-coupled receptor trafficking.Handb Exp Pharmacol. 2014;219:173-86. doi: 10.1007/978-3-642-41199-1_9. Handb Exp Pharmacol. 2014. PMID: 24292830 Free PMC article. Review.
-
Signal transduction at GPCRs: Allosteric activation of the ERK MAPK by β-arrestin.Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2303794120. doi: 10.1073/pnas.2303794120. Epub 2023 Oct 16. Proc Natl Acad Sci U S A. 2023. PMID: 37844230 Free PMC article.
-
Screening β-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors.J Biomol Screen. 2013 Jun;18(5):599-609. doi: 10.1177/1087057113475480. Epub 2013 Feb 8. J Biomol Screen. 2013. PMID: 23396314
-
Subcellular Organization of GPCR Signaling.Trends Pharmacol Sci. 2018 Feb;39(2):200-208. doi: 10.1016/j.tips.2017.11.009. Epub 2018 Jan 28. Trends Pharmacol Sci. 2018. PMID: 29478570 Free PMC article. Review.
Cited by
-
Versatility of 14-3-3 proteins and their roles in bone and joint-related diseases.Bone Res. 2024 Oct 15;12(1):58. doi: 10.1038/s41413-024-00370-4. Bone Res. 2024. PMID: 39406741 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

