Rational synthesis of elusive organic-inorganic hybrid metal-oxo clusters: formation and post-functionalization of hexavanadates
- PMID: 37234890
- PMCID: PMC10207889
- DOI: 10.1039/d3sc00038a
Rational synthesis of elusive organic-inorganic hybrid metal-oxo clusters: formation and post-functionalization of hexavanadates
Abstract
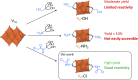
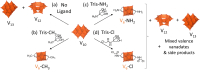
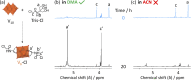
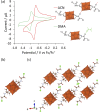
Paving the way towards new functional materials relies increasingly on the challenging task of forming organic-inorganic hybrid compounds. In that regard, discrete atomically-precise metal-oxo nanoclusters have received increasing attention due to the wide range of organic moieties that can be grafted onto them through functionalization reactions. The Lindqvist hexavanadate family of clusters, such as [V6O13{(OCH2)3C-R}2]2- (V6-R), is particularly interesting due to the magnetic, redox, and catalytic properties of these clusters. However, compared to other metal-oxo cluster types, V6-R clusters have been less extensively explored, which is mainly due to poorly understood synthetic challenges and the limited number of viable post-functionalization strategies. In this work, we present an in-depth investigation of the factors that influence the formation of hybrid hexavanadates (V6-R HPOMs) and leverage this knowledge to develop [V6O13{(OCH2)3CNHCOCH2Cl}2]2- (V6-Cl) as a new and tunable platform for the facile formation of discrete hybrid structures based on metal-oxo clusters in relatively high yields. Moreover, we showcase the versatility of the V6-Cl platform through its post-functionalization via nucleophilic substitution with various carboxylic acids of differing complexity and with functionalities that are relevant in multiple disciplines, such as supramolecular chemistry and biochemistry. Hence, V6-Cl was shown to be a straightforward and versatile starting point for the formation of functional supramolecular structures or other hybrid materials, thereby enabling their exploration in various fields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Versatile post-functionalisation strategy for the formation of modular organic-inorganic polyoxometalate hybrids.Chem Sci. 2022 Feb 8;13(10):2891-2899. doi: 10.1039/d1sc06326j. eCollection 2022 Mar 9. Chem Sci. 2022. PMID: 35382468 Free PMC article.
-
Versatile Organic Chemistry on Vanadium-Based Multi-Electron Reservoirs.Chemistry. 2018 Feb 21;24(11):2785-2789. doi: 10.1002/chem.201800041. Epub 2018 Feb 6. Chemistry. 2018. PMID: 29314364
-
Experimental and theoretical insights of functionalized hexavanadates on Na+/K+-ATPase activity; molecular interaction field, ab initio calculations and in vitro assays.J Inorg Biochem. 2019 Sep;198:110720. doi: 10.1016/j.jinorgbio.2019.110720. Epub 2019 May 16. J Inorg Biochem. 2019. PMID: 31150927
-
Reactivity of metal-oxo clusters towards biomolecules: from discrete polyoxometalates to metal-organic frameworks.Chem Soc Rev. 2024 Jan 2;53(1):84-136. doi: 10.1039/d3cs00195d. Chem Soc Rev. 2024. PMID: 38015569 Review.
-
Platonic and Archimedean solids in discrete metal-containing clusters.Chem Soc Rev. 2023 Jan 3;52(1):383-444. doi: 10.1039/d2cs00582d. Chem Soc Rev. 2023. PMID: 36533405 Review.
Cited by
-
Bioorthogonal chemistry of polyoxometalates - challenges and prospects.Chem Sci. 2024 Feb 26;15(12):4202-4221. doi: 10.1039/d3sc06284h. eCollection 2024 Mar 20. Chem Sci. 2024. PMID: 38516091 Free PMC article. Review.
References
-
- Zhang J. Huang Y. Li G. Wei Y. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2019;378:395–414. doi: 10.1016/j.ccr.2017.10.025. - DOI
-
- Zhang Y. de Azambuja F. Parac-Vogt T. N. The forgotten chemistry of group(IV) metals: A survey on the synthesis, structure, and properties of discrete Zr(IV), Hf(IV), and Ti(IV) oxo clusters. Coord. Chem. Rev. 2021;438:213886. doi: 10.1016/j.ccr.2021.213886. - DOI
-
- Song Y.-F., Polyoxometalate-Based Assemblies and Functional Materials, Springer International Publishing, Cham, 2018, vol. 176
LinkOut - more resources
Full Text Sources

