The role of blood flow in vessel remodeling and its regulatory mechanism during developmental angiogenesis
- PMID: 37221410
- PMCID: PMC11072276
- DOI: 10.1007/s00018-023-04801-z
The role of blood flow in vessel remodeling and its regulatory mechanism during developmental angiogenesis
Abstract
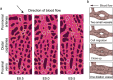
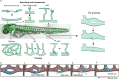
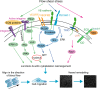
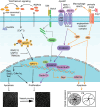
Vessel remodeling is essential for a functional and mature vascular network. According to the difference in endothelial cell (EC) behavior, we classified vessel remodeling into vessel pruning, vessel regression and vessel fusion. Vessel remodeling has been proven in various organs and species, such as the brain vasculature, subintestinal veins (SIVs), and caudal vein (CV) in zebrafish and yolk sac vessels, retina, and hyaloid vessels in mice. ECs and periendothelial cells (such as pericytes and astrocytes) contribute to vessel remodeling. EC junction remodeling and actin cytoskeleton dynamic rearrangement are indispensable for vessel pruning. More importantly, blood flow has a vital role in vessel remodeling. In recent studies, several mechanosensors, such as integrins, platelet endothelial cell adhesion molecule-1 (PECAM-1)/vascular endothelial cell (VE-cadherin)/vascular endothelial growth factor receptor 2 (VEGFR2) complex, and notch1, have been shown to contribute to mechanotransduction and vessel remodeling. In this review, we highlight the current knowledge of vessel remodeling in mouse and zebrafish models. We further underline the contribution of cellular behavior and periendothelial cells to vessel remodeling. Finally, we discuss the mechanosensory complex in ECs and the molecular mechanisms responsible for vessel remodeling.
Keywords: EC rearrangement; Hemodynamics; Vessel pruning; Wnt signaling; Zebrafish.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no relevant financial interests to disclose.
Figures


Similar articles
-
The blood flow-klf6a-tagln2 axis drives vessel pruning in zebrafish by regulating endothelial cell rearrangement and actin cytoskeleton dynamics.PLoS Genet. 2021 Jul 28;17(7):e1009690. doi: 10.1371/journal.pgen.1009690. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34319989 Free PMC article.
-
Apoptosis of Endothelial Cells Contributes to Brain Vessel Pruning of Zebrafish During Development.Front Mol Neurosci. 2018 Jun 28;11:222. doi: 10.3389/fnmol.2018.00222. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30002618 Free PMC article.
-
Cell-cell junctional mechanotransduction in endothelial remodeling.Cell Mol Life Sci. 2017 Jan;74(2):279-292. doi: 10.1007/s00018-016-2325-8. Epub 2016 Aug 9. Cell Mol Life Sci. 2017. PMID: 27506620 Free PMC article. Review.
-
Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis.Development. 2014 Apr;141(8):1757-66. doi: 10.1242/dev.104422. Development. 2014. PMID: 24715464
-
Vascular remodeling: A redox-modulated mechanism of vessel caliber regulation.Free Radic Biol Med. 2017 Aug;109:11-21. doi: 10.1016/j.freeradbiomed.2017.01.025. Epub 2017 Jan 18. Free Radic Biol Med. 2017. PMID: 28109889 Review.
Cited by
-
BRAF Modulates the Interplay Between Cell-Cell and Cell-Extracellular Matrix Adhesions in PECAM-1-Mediated Mechanotransduction.Int J Mol Sci. 2024 Oct 18;25(20):11234. doi: 10.3390/ijms252011234. Int J Mol Sci. 2024. PMID: 39457016 Free PMC article.
-
Plxnd1-mediated mechanosensing of blood flow controls the caliber of the Dorsal Aorta via the transcription factor Klf2.bioRxiv [Preprint]. 2024 Jan 25:2024.01.24.576555. doi: 10.1101/2024.01.24.576555. bioRxiv. 2024. PMID: 38328196 Free PMC article. Preprint.
-
Oxygen generating biomaterials at the forefront of regenerative medicine: advances in bone regeneration.Front Bioeng Biotechnol. 2024 Jan 12;12:1292171. doi: 10.3389/fbioe.2024.1292171. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38282892 Free PMC article. Review.
-
The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment.Molecules. 2023 Jul 19;28(14):5500. doi: 10.3390/molecules28145500. Molecules. 2023. PMID: 37513375 Free PMC article. Review.
-
Decoding Tumor Angiogenesis for Therapeutic Advancements: Mechanistic Insights.Biomedicines. 2024 Apr 9;12(4):827. doi: 10.3390/biomedicines12040827. Biomedicines. 2024. PMID: 38672182 Free PMC article. Review.
References
-
- Suzanne H, Huijun Y, Tailoi C-L. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41(5):1217–1228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

