Correlative super-resolution analysis of cardiac calcium sparks and their molecular origins in health and disease
- PMID: 37220792
- PMCID: PMC10205181
- DOI: 10.1098/rsob.230045
Correlative super-resolution analysis of cardiac calcium sparks and their molecular origins in health and disease
Abstract
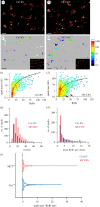
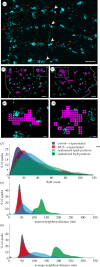
Rapid release of calcium from internal stores via ryanodine receptors (RyRs) is one of the fastest types of cytoplasmic second messenger signalling in excitable cells. In the heart, rapid summation of the elementary events of calcium release, 'calcium sparks', determine the contraction of the myocardium. We adapted a correlative super-resolution microscopy protocol to correlate sub-plasmalemmal spontaneous calcium sparks in rat right ventricular myocytes with the local nanoscale RyR2 positions. This revealed a steep relationship between the integral of a calcium spark and the sum of the local RyR2s. Segmentation of recurring spark sites showed evidence of repeated and triggered saltatory activation of multiple local RyR2 clusters. In myocytes taken from failing right ventricles, RyR2 clusters themselves showed a dissipated morphology and fragmented (smaller) clusters. They also featured greater heterogeneity in both the spark properties and the relationship between the integral of the calcium spark and the local ensemble of RyR2s. While fragmented (smaller) RyR2 clusters were rarely observed directly underlying the larger sparks or the recurring spark sites, local interrogation of the channel-to-channel distances confirmed a clear link between the positions of each calcium spark and the tight, non-random clustering of the local RyR2 in both healthy and failing ventricles.
Keywords: DNA-PAINT; calcium signalling; correlative light microscopy; nanodomains; ryanodine receptor.
Conflict of interest statement
Authors declare no competing interests.
Figures

Similar articles
-
Functional groups of ryanodine receptors in rat ventricular cells.J Physiol. 2007 Aug 15;583(Pt 1):251-69. doi: 10.1113/jphysiol.2007.136549. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627991 Free PMC article.
-
Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling.J Mol Cell Cardiol. 2016 Mar;92:82-92. doi: 10.1016/j.yjmcc.2016.01.024. Epub 2016 Jan 28. J Mol Cell Cardiol. 2016. PMID: 26827896 Free PMC article.
-
β2-adrenergic stimulation potentiates spontaneous calcium release by increasing signal mass and co-activation of ryanodine receptor clusters.Acta Physiol (Oxf). 2022 Apr;234(4):e13736. doi: 10.1111/apha.13736. Epub 2021 Nov 6. Acta Physiol (Oxf). 2022. PMID: 34709723
-
Elementary calcium release events from the sarcoplasmic reticulum in the heart.Adv Exp Med Biol. 2012;740:499-509. doi: 10.1007/978-94-007-2888-2_21. Adv Exp Med Biol. 2012. PMID: 22453956 Free PMC article. Review.
-
Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells.Front Biosci. 2002 Sep 1;7:d1867-78. doi: 10.2741/A885. Front Biosci. 2002. PMID: 12161336 Review.
Cited by
-
Decoding life's inner workings: advances in quantitative bioimaging.Open Biol. 2023 Nov;13(11):230329. doi: 10.1098/rsob.230329. Epub 2023 Nov 29. Open Biol. 2023. PMID: 38018093 Free PMC article.
-
Propagation of conformational instability in FK506-binding protein FKBP12.Biochim Biophys Acta Proteins Proteom. 2024 May 1;1872(3):140990. doi: 10.1016/j.bbapap.2023.140990. Epub 2023 Dec 23. Biochim Biophys Acta Proteins Proteom. 2024. PMID: 38142946
-
Super-Resolution Analysis of the Origins of the Elementary Events of ER Calcium Release in Dorsal Root Ganglion Neurons.Cells. 2023 Dec 23;13(1):38. doi: 10.3390/cells13010038. Cells. 2023. PMID: 38201242 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

