Inhibition of the extracellular enzyme A disintegrin and metalloprotease with thrombospondin motif 4 prevents cardiac fibrosis and dysfunction
- PMID: 37216909
- PMCID: PMC10439713
- DOI: 10.1093/cvr/cvad078
Inhibition of the extracellular enzyme A disintegrin and metalloprotease with thrombospondin motif 4 prevents cardiac fibrosis and dysfunction
Abstract
Aims: Heart failure is a condition with high mortality rates, and there is a lack of therapies that directly target maladaptive changes in the extracellular matrix (ECM), such as fibrosis. We investigated whether the ECM enzyme known as A disintegrin and metalloprotease with thrombospondin motif (ADAMTS) 4 might serve as a therapeutic target in treatment of heart failure and cardiac fibrosis.
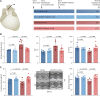
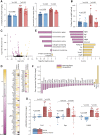
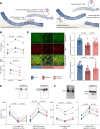
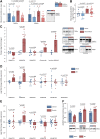
Methods and results: The effects of pharmacological ADAMTS4 inhibition on cardiac function and fibrosis were examined in rats exposed to cardiac pressure overload. Disease mechanisms affected by the treatment were identified based on changes in the myocardial transcriptome. Following aortic banding, rats receiving an ADAMTS inhibitor, with high inhibitory capacity for ADAMTS4, showed substantially better cardiac function than vehicle-treated rats, including ∼30% reduction in E/e' and left atrial diameter, indicating an improvement in diastolic function. ADAMTS inhibition also resulted in a marked reduction in myocardial collagen content and a down-regulation of transforming growth factor (TGF)-β target genes. The mechanism for the beneficial effects of ADAMTS inhibition was further studied in cultured human cardiac fibroblasts producing mature ECM. ADAMTS4 caused a 50% increase in the TGF-β levels in the medium. Simultaneously, ADAMTS4 elicited a not previously known cleavage of TGF-β-binding proteins, i.e. latent-binding protein of TGF-β and extra domain A-fibronectin. These effects were abolished by the ADAMTS inhibitor. In failing human hearts, we observed a marked increase in ADAMTS4 expression and cleavage activity.
Conclusion: Inhibition of ADAMTS4 improves cardiac function and reduces collagen accumulation in rats with cardiac pressure overload, possibly through a not previously known cleavage of molecules that control TGF-β availability. Targeting ADAMTS4 may serve as a novel strategy in heart failure treatment, in particular, in heart failure with fibrosis and diastolic dysfunction.
Keywords: ADAMTS enzymes; Cardiac fibrosis; Extracellular matrix; Heart failure; New therapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A patent application filed by the University of Oslo covering the use of ADAMTS4 inhibition in cardiac remodelling and heart failure (WO2015004209A1) is pending in Europe (EP3756667A1) and granted in the United States (US10744155B2). The inventors named in this patent are among the authors (M.V., G.C., J.M.A., I.S., I.G.L., and C.R.C.). The ADAMTS inhibitor used in this study was supplied free of charge by AstraZeneca under a material transfer agreement. A licence agreement with the pharmaceutical company Paradigm Biopharma for the development of pentosane polysulfate (PPS) in the treatment of cardiac remodelling and heart failure was signed between the University of Oslo and Paradigm in 2017, and a patent for the use of PPS in heart failure has been filed by Paradigm Biopharma (PCT/AU2022/051301) where M.V. and G.C. are among the inventors. M.V. has performed consulting services for Paradigm to support this development. The PPS was not used in this study. M.V. has participated in advisory board for Pharmacosmos (iron supplementation in heart failure).
Figures


Similar articles
-
Pentosan polysulfate decreases myocardial expression of the extracellular matrix enzyme ADAMTS4 and improves cardiac function in vivo in rats subjected to pressure overload by aortic banding.PLoS One. 2014 Mar 3;9(3):e89621. doi: 10.1371/journal.pone.0089621. eCollection 2014. PLoS One. 2014. PMID: 24595230 Free PMC article.
-
MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure.Hypertension. 2018 Feb;71(2):280-288. doi: 10.1161/HYPERTENSIONAHA.117.10094. Epub 2017 Dec 18. Hypertension. 2018. PMID: 29255073
-
Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5.J Mol Cell Cardiol. 2014 Nov;76:148-57. doi: 10.1016/j.yjmcc.2014.08.008. Epub 2014 Aug 26. J Mol Cell Cardiol. 2014. PMID: 25169971
-
The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family.Genome Biol. 2015 May 30;16(1):113. doi: 10.1186/s13059-015-0676-3. Genome Biol. 2015. PMID: 26025392 Free PMC article. Review.
-
A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease.Biochem Pharmacol. 2019 Jun;164:188-204. doi: 10.1016/j.bcp.2019.03.033. Epub 2019 Mar 21. Biochem Pharmacol. 2019. PMID: 30905657 Free PMC article. Review.
Cited by
-
Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Perspective from Fibroblasts.Int J Mol Sci. 2023 Oct 2;24(19):14845. doi: 10.3390/ijms241914845. Int J Mol Sci. 2023. PMID: 37834293 Free PMC article. Review.
-
ADAMTS4 is a crucial proteolytic enzyme for versican cleavage in the amnion at parturition.Commun Biol. 2024 Mar 9;7(1):301. doi: 10.1038/s42003-024-06007-w. Commun Biol. 2024. PMID: 38461223 Free PMC article.
-
Hitting the Target! Challenges and Opportunities for TGF-β Inhibition for the Treatment of Cardiac fibrosis.Pharmaceuticals (Basel). 2024 Feb 20;17(3):267. doi: 10.3390/ph17030267. Pharmaceuticals (Basel). 2024. PMID: 38543053 Free PMC article. Review.
-
Temporal expression and spatial distribution of the proteoglycan versican during cardiac fibrosis development.Matrix Biol Plus. 2023 Nov 10;19-20:100135. doi: 10.1016/j.mbplus.2023.100135. eCollection 2023 Dec. Matrix Biol Plus. 2023. PMID: 38076279 Free PMC article.
-
Hypertensive Heart Disease: Mechanisms, Diagnosis and Treatment.Rev Cardiovasc Med. 2024 Mar 6;25(3):93. doi: 10.31083/j.rcm2503093. eCollection 2024 Mar. Rev Cardiovasc Med. 2024. PMID: 39076964 Free PMC article. Review.
References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW. Heart disease and stroke statistics—2021 update. Circulation 2021;143:e254–e743. - PubMed
-
- Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

