Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson's disease
- PMID: 37214398
- PMCID: PMC10196133
- DOI: 10.3389/fnins.2023.1146979
Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson's disease
Abstract
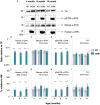
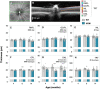
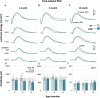
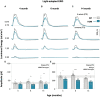
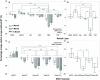
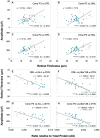
Abnormal alpha-synuclein (α-SYN) protein deposition has long been recognized as one of the pathological hallmarks of Parkinson's disease's (PD). This study considers the potential utility of PD retinal biomarkers by investigating retinal changes in a well characterized PD model of α-SYN overexpression and how these correspond to the presence of retinal α-SYN. Transgenic A53T homozygous (HOM) mice overexpressing human α-SYN and wildtype (WT) control littermates were assessed at 4, 6, and 14 months of age (male and female, n = 15-29 per group). In vivo retinal function (electroretinography, ERG) and structure (optical coherence tomography, OCT) were recorded, and retinal immunohistochemistry and western blot assays were performed to examine retinal α-SYN and tyrosine hydroxylase. Compared to WT controls, A53T mice exhibited reduced light-adapted (cone photoreceptor and bipolar cell amplitude, p < 0.0001) ERG responses and outer retinal thinning (outer plexiform layer, outer nuclear layer, p < 0.0001) which correlated with elevated levels of α-SYN. These retinal signatures provide a high throughput means to study α-SYN induced neurodegeneration and may be useful in vivo endpoints for PD drug discovery.
Keywords: A53T; Parkinson’s disease; alpha-synuclein; electroretinography; optical coherence tomography; retina.
Copyright © 2023 Tran, Wong, Hoang, Finkelstein, Bui and Nguyen.
Conflict of interest statement
CN and BB are joint investigators on an Australian Research Council Linkage grant LP160100126 with AstraZeneca Neuroscience and Biogen Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson's Disease.Biomedicines. 2024 Jan 8;12(1):130. doi: 10.3390/biomedicines12010130. Biomedicines. 2024. PMID: 38255235 Free PMC article.
-
Age-dependent neurodegeneration and neuroinflammation in a genetic A30P/A53T double-mutated α-synuclein mouse model of Parkinson's disease.Neurobiol Dis. 2022 Sep;171:105798. doi: 10.1016/j.nbd.2022.105798. Epub 2022 Jun 21. Neurobiol Dis. 2022. PMID: 35750147
-
Accelerated accumulation of retinal α-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson's disease.Neurobiol Dis. 2019 Jan;121:1-16. doi: 10.1016/j.nbd.2018.09.013. Epub 2018 Sep 12. Neurobiol Dis. 2019. PMID: 30218757
-
Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson's disease.Glia. 2018 Aug;66(8):1752-1762. doi: 10.1002/glia.23338. Epub 2018 Apr 6. Glia. 2018. PMID: 29624735
-
Silencing of Glucocerebrosidase Gene in Drosophila Enhances the Aggregation of Parkinson's Disease Associated α-Synuclein Mutant A53T and Affects Locomotor Activity.Front Neurosci. 2018 Feb 16;12:81. doi: 10.3389/fnins.2018.00081. eCollection 2018. Front Neurosci. 2018. PMID: 29503608 Free PMC article.
Cited by
-
Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson's Disease.Biomedicines. 2024 Jan 8;12(1):130. doi: 10.3390/biomedicines12010130. Biomedicines. 2024. PMID: 38255235 Free PMC article.
-
Alpha Synuclein Toxicity and Non-Motor Parkinson's.Cells. 2024 Jul 27;13(15):1265. doi: 10.3390/cells13151265. Cells. 2024. PMID: 39120295 Free PMC article. Review.
-
Retinal electrophysiology in central nervous system disorders. A review of human and mouse studies.Front Neurosci. 2023 Aug 2;17:1215097. doi: 10.3389/fnins.2023.1215097. eCollection 2023. Front Neurosci. 2023. PMID: 37600004 Free PMC article. Review.
-
Assessment of Changes in Vascular Density in the Layers of the Eye in Patients With Parkinson's Disease.Ann Neurosci. 2024 Sep 3:09727531241259841. doi: 10.1177/09727531241259841. Online ahead of print. Ann Neurosci. 2024. PMID: 39544663 Free PMC article.
-
Retinal hyperspectral imaging in mouse models of Parkinson's disease and healthy aging.Sci Rep. 2024 Jul 12;14(1):16089. doi: 10.1038/s41598-024-66284-7. Sci Rep. 2024. PMID: 38997314 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

