Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes
- PMID: 37209944
- PMCID: PMC10325927
- DOI: 10.1016/j.canlet.2023.216220
Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes
Abstract
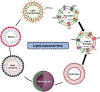
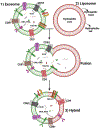
Cancer has recently surpassed heart disease as the leading cause of deaths worldwide for the age group 45-65 and has been the primary focus for biomedical researchers. Presently, the drugs involved in the first-line cancer therapy are raising concerns due to high toxicity and lack of selectivity to cancer cells. There has been a significant increase in research with innovative nano formulations to entrap the therapeutic payload to enhance efficacy and eliminate or minimize toxic effects. Lipid-based carriers stand out due to their unique structural properties and biocompatible nature. The two main leaders of lipid-based drug carriers: long known liposomes and comparatively new exosomes have been well-researched. The similarity between the two lipid-based carriers is the vesicular structure with the core's capability to carry the payload. While liposomes utilize chemically derived and altered phospholipid components, the exosomes are naturally occurring vesicles with inherent lipids, proteins, and nucleic acids. More recently, researchers have focused on developing hybrid exosomes by fusing liposomes and exosomes. Combining these two types of vesicles may offer some advantages such as high drug loading, targeted cellular uptake, biocompatibility, controlled release, stability in harsh conditions and low immunogenicity.
Keywords: Cancer therapeutics; Exosomes; Fusion; Hybrid exosomes; Lipid-based vesicles; Liposomes.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Dr. Ramesh C. Gupta holds positions both at the University of Louisville and 3P Biotechnologies, Inc. The authors have filed an international patent application (PCT) based on part of the results reported in this paper. All other authors declare no conflict of interest.
Figures

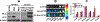
Similar articles
-
Milk/colostrum exosomes: A nanoplatform advancing delivery of cancer therapeutics.Cancer Lett. 2023 May 1;561:216141. doi: 10.1016/j.canlet.2023.216141. Epub 2023 Mar 22. Cancer Lett. 2023. PMID: 36963459 Free PMC article. Review.
-
Innovative Phospholipid Carriers: A Viable Strategy to Counteract Antimicrobial Resistance.Int J Mol Sci. 2023 Nov 3;24(21):15934. doi: 10.3390/ijms242115934. Int J Mol Sci. 2023. PMID: 37958915 Free PMC article. Review.
-
Exosome mimetics: a novel class of drug delivery systems.Int J Nanomedicine. 2012;7:1525-41. doi: 10.2147/IJN.S29661. Epub 2012 Mar 16. Int J Nanomedicine. 2012. PMID: 22619510 Free PMC article. Review.
-
Targeted si-RNA with liposomes and exosomes (extracellular vesicles): How to unlock the potential.Int J Pharm. 2017 Jun 20;525(2):293-312. doi: 10.1016/j.ijpharm.2017.01.056. Epub 2017 Feb 3. Int J Pharm. 2017. PMID: 28163221 Review.
-
Research progress of exosomes as drug carriers in cancer and inflammation.J Drug Target. 2023 Apr;31(4):335-353. doi: 10.1080/1061186X.2022.2162059. Epub 2023 Jan 3. J Drug Target. 2023. PMID: 36543743
Cited by
-
TfR Aptamer-Functionalized MSNs for Enhancing Targeted Cellular Uptake and Therapy of Cancer Cells.ACS Omega. 2023 Dec 12;8(51):48975-48983. doi: 10.1021/acsomega.3c06562. eCollection 2023 Dec 26. ACS Omega. 2023. PMID: 38162791 Free PMC article.
-
Exosomes as emerging nanoplatform in cancer therapy.Cancer Lett. 2023 Oct 10;574:216394. doi: 10.1016/j.canlet.2023.216394. Epub 2023 Sep 15. Cancer Lett. 2023. PMID: 37716464 Free PMC article. No abstract available.
-
Extracellular Vesicle MicroRNAs in Heart Failure: Pathophysiological Mediators and Therapeutic Targets.Cells. 2023 Aug 25;12(17):2145. doi: 10.3390/cells12172145. Cells. 2023. PMID: 37681877 Free PMC article. Review.
-
Extracellular vesicles as the next-generation modulators of pharmacokinetics and pharmacodynamics of medications and their potential as adjuvant therapeutics.Clin Transl Med. 2024 Aug;14(8):e70002. doi: 10.1002/ctm2.70002. Clin Transl Med. 2024. PMID: 39167024 Free PMC article. Review.
-
Cancer/Testis Antigens as Targets for RNA-Based Anticancer Therapy.Int J Mol Sci. 2023 Sep 28;24(19):14679. doi: 10.3390/ijms241914679. Int J Mol Sci. 2023. PMID: 37834126 Free PMC article. Review.
References
-
- Lin A, Giuliano CJ, Palladino A, John KM, Abramowicz C, Yuan ML, Sausville EL, Lukow DA, Liu L, Chait AR, Galluzzo ZC, Tucker C, Sheltzer JM, Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials, Science Translational Medicine, 11 (2019) eaaw8412. - PMC - PubMed
-
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date, Pharm Res, 33 (2016) 2373–2387. - PubMed
-
- Medina-Alarcón KP, Voltan AR, Fonseca-Santos B, Moro IJ, De Oliveira Souza F, Chorilli M, Soares CP, Dos Santos AG, Mendes-Giannini MJS, Fusco-Almeida AM, Highlights in nanocarriers for the treatment against cervical cancer, Materials Science and Engineering: C, 80 (2017) 748–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

