Myo1f has an essential role in γδT intraepithelial lymphocyte adhesion and migration
- PMID: 37207213
- PMCID: PMC10189005
- DOI: 10.3389/fimmu.2023.1041079
Myo1f has an essential role in γδT intraepithelial lymphocyte adhesion and migration
Abstract
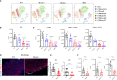
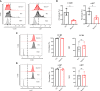
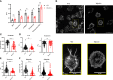
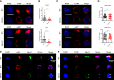
γδT intraepithelial lymphocyte represents up to 60% of the small intestine intraepithelial compartment. They are highly migrating cells and constantly interact with the epithelial cell layer and lamina propria cells. This migratory phenotype is related to the homeostasis of the small intestine, the control of bacterial and parasitic infections, and the epithelial shedding induced by LPS. Here, we demonstrate that Myo1f participates in the adhesion and migration of intraepithelial lymphocytes. Using long-tailed class I myosins KO mice, we identified the requirement of Myo1f for their migration to the small intestine intraepithelial compartment. The absence of Myo1f affects intraepithelial lymphocytes' homing due to reduced CCR9 and α4β7 surface expression. In vitro, we confirm that adhesion to integrin ligands and CCL25-dependent and independent migration of intraepithelial lymphocytes are Myo1f-dependent. Mechanistically, Myo1f deficiency prevents correct chemokine receptor and integrin polarization, leading to reduced tyrosine phosphorylation which could impact in signal transduction. Overall, we demonstrate that Myo1f has an essential role in the adhesion and migration in γδT intraepithelial lymphocytes.
Keywords: class I myosins; cytoskeleton; integrins; intraepithelial lymphocytes; migration; signaling.
Copyright © 2023 Martínez-Vargas, Sánchez-Bello, Miguel-Rodríguez, Hernández-Cázares, Santos-Argumedo and Talamás-Rohana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Myo1f, an Unconventional Long-Tailed Myosin, Is a New Partner for the Adaptor 3BP2 Involved in Mast Cell Migration.Front Immunol. 2019 May 9;10:1058. doi: 10.3389/fimmu.2019.01058. eCollection 2019. Front Immunol. 2019. PMID: 31143189 Free PMC article.
-
Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn's disease.Gastroenterology. 2008 Jun;134(7):2025-35. doi: 10.1053/j.gastro.2008.02.085. Epub 2008 Mar 5. Gastroenterology. 2008. PMID: 18439426
-
Intrarectal immunization and IgA antibody-secreting cell homing to the small intestine.J Immunol. 2013 May 1;190(9):4836-47. doi: 10.4049/jimmunol.1202979. Epub 2013 Apr 1. J Immunol. 2013. PMID: 23547118
-
Long-Tailed Unconventional Class I Myosins in Health and Disease.Int J Mol Sci. 2020 Apr 7;21(7):2555. doi: 10.3390/ijms21072555. Int J Mol Sci. 2020. PMID: 32272642 Free PMC article. Review.
-
Role of beta7 integrins in intestinal lymphocyte homing and retention.Curr Mol Med. 2009 Sep;9(7):836-50. doi: 10.2174/156652409789105525. Curr Mol Med. 2009. PMID: 19860663 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

