Association between the expression levels of ADAMTS16 and BMP2 and tumor budding in hepatocellular carcinoma
- PMID: 37205917
- PMCID: PMC10189853
- DOI: 10.3892/ol.2023.13842
Association between the expression levels of ADAMTS16 and BMP2 and tumor budding in hepatocellular carcinoma
Abstract
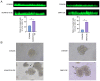
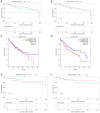
Tumor budding (TB) has become a crucial factor for predicting the malignancy grade and prognostic outcome for multiple types of solid cancer. Studies have investigated the prognostic value of TB in hepatocellular carcinoma (HCC). However, its molecular mechanism in HCC remains unclear. To the best of our knowledge, the present study was the first to compare the expression of differentially expressed genes (DEGs) between TB-positive (TB-pos) and TB-negative HCC tissues. In the present study, total RNA was extracted from 40 HCC tissue specimens and then sequenced. According to Gene Ontology (GO) functional annotation, upregulated DEGs were markedly associated with embryonic kidney development-related GO terms, which suggested that the TB process may at least partly mimic the process of embryonic kidney development. Subsequently, two genes, a disintegrin and metalloproteinase domain with thrombospondin motifs 16 (ADAMTS16) and bone morphogenetic protein 2 (BMP2), were screened and verified through immunohistochemical analysis of HCC tissue microarrays. According to the immunohistochemical results, ADAMTS16 and BMP2 were upregulated in TB-pos HCC samples, and BMP2 expression was increased in budding cells compared with the tumor center. Additionally, through cell culture experiments, it was demonstrated that ADAMTS16 and BMP2 may promote TB of liver cancer, thus promoting the malignant progression of liver cancer. Further analysis revealed that ADAMTS16 expression was associated with necrosis and cholestasis, and BMP2 expression was associated with the Barcelona Clinic Liver Cancer stage and the vessels encapsulating tumor clusters. Overall, the findings of the present study provided insights into the possible mechanisms of TB in HCC and revealed potential anti-HCC therapeutic targets.
Keywords: a metalloproteinase domain with thrombospondin motifs 16; bone morphogenetic protein 2; hepatocellular carcinoma; molecular mechanism; tumor budding.
Copyright: © Jiang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Inhibition of Bone Morphogenetic Protein 2 Suppresses the Stemness Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma via the MAPK/ERK Pathway.Cancer Manag Res. 2021 Jan 27;13:773-785. doi: 10.2147/CMAR.S281969. eCollection 2021. Cancer Manag Res. 2021. PMID: 33536785 Free PMC article.
-
A classification based on tumor budding and immune score for patients with hepatocellular carcinoma.Oncoimmunology. 2019 Nov 7;9(1):1672495. doi: 10.1080/2162402X.2019.1672495. eCollection 2020. Oncoimmunology. 2019. PMID: 32002283 Free PMC article.
-
BMP2 secretion from hepatocellular carcinoma cell HepG2 enhances angiogenesis and tumor growth in endothelial cells via activation of the MAPK/p38 signaling pathway.Stem Cell Res Ther. 2019 Aug 6;10(1):237. doi: 10.1186/s13287-019-1301-2. Stem Cell Res Ther. 2019. Retraction in: Stem Cell Res Ther. 2022 Apr 08;13(1):154. doi: 10.1186/s13287-022-02841-z PMID: 31387619 Free PMC article. Retracted.
-
Targeting BMP2 for therapeutic strategies against hepatocellular carcinoma.Transl Oncol. 2024 Aug;46:101970. doi: 10.1016/j.tranon.2024.101970. Epub 2024 May 25. Transl Oncol. 2024. PMID: 38797016 Free PMC article.
-
Tumor budding as a potential prognostic marker in determining the behavior of primary liver cancers.World J Hepatol. 2023 Jun 27;15(6):775-785. doi: 10.4254/wjh.v15.i6.775. World J Hepatol. 2023. PMID: 37397937 Free PMC article. Review.
Cited by
-
Predictors of Local Invasion in Infiltrative Basal Cell Carcinoma: Tumour Budding Outperforms the WHO Subtyping.Acta Derm Venereol. 2024 Jul 2;104:adv40172. doi: 10.2340/actadv.v104.40172. Acta Derm Venereol. 2024. PMID: 38956962 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
