This is a preprint.
The lncRNA Neat1 is associated with astrocyte reactivity and memory deficits in a mouse model of Alzheimer's disease
- PMID: 37205548
- PMCID: PMC10187170
- DOI: 10.1101/2023.05.03.539260
The lncRNA Neat1 is associated with astrocyte reactivity and memory deficits in a mouse model of Alzheimer's disease
Abstract
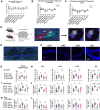
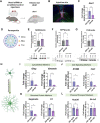
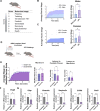
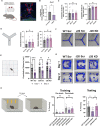
Dysregulation of long non-coding RNAs (lncRNAs) have been associated with Alzheimer's disease (AD). However, the functional role of lncRNAs in AD remains unclear. Here, we report a crucial role for the lncRNA Neat1 in astrocyte dysfunction and memory deficits associated with AD. Transcriptomics analysis show abnormally high expression levels of NEAT1 in the brains of AD patients relative to aged-matched healthy controls, with the most significantly elevated levels in glial cells. In a human transgenic APP-J20 (J20) mouse model of AD, RNA-fluorescent in situ hybridization characterization of Neat1 expression in hippocampal astrocyte versus non-astrocyte cell populations revealed a significant increase in Neat1 expression in astrocytes of male, but not female, mice. This corresponded with increased seizure susceptibility in J20 male mice. Interestingly, Neat1 deficiency in the dCA1 in J20 male mice did not alter seizure threshold. Mechanistically, Neat1 deficiency in the dorsal area CA1 of the hippocampus (dCA1) J20 male mice significantly improved hippocampus-dependent memory. Neat1 deficiency also remarkably reduced astrocyte reactivity markers suggesting that Neat1 overexpression is associated with astrocyte dysfunction induced by hAPP/Aβ in the J20 mice. Together, these findings indicate that abnormal Neat1 overexpression may contribute to memory deficits in the J20 AD model not through altered neuronal activity, but through astrocyte dysfunction.
Conflict of interest statement
Competing Interests All authors declare no competing interests.
Figures
Similar articles
-
The Time Course of Recognition Memory Impairment and Glial Pathology in the hAPP-J20 Mouse Model of Alzheimer's Disease.J Alzheimers Dis. 2019;68(2):609-624. doi: 10.3233/JAD-181238. J Alzheimers Dis. 2019. PMID: 30814360
-
Downregulation of Dickkopf-3, a Wnt antagonist elevated in Alzheimer's disease, restores synapse integrity and memory in a disease mouse model.Elife. 2024 Jan 29;12:RP89453. doi: 10.7554/eLife.89453. Elife. 2024. PMID: 38285009 Free PMC article.
-
Age-dependent alterations of the hippocampal cell composition and proliferative potential in the hAβPPSwInd-J20 mouse.J Alzheimers Dis. 2014;41(4):1177-92. doi: 10.3233/JAD-132717. J Alzheimers Dis. 2014. PMID: 24787919
-
Whole-body vibration ameliorates glial pathological changes in the hippocampus of hAPP transgenic mice, but does not affect plaque load.Behav Brain Funct. 2023 Mar 20;19(1):5. doi: 10.1186/s12993-023-00208-9. Behav Brain Funct. 2023. PMID: 36941713 Free PMC article.
-
Phenotypic Differences between the Alzheimer's Disease-Related hAPP-J20 Model and Heterozygous Zbtb20 Knock-Out Mice.eNeuro. 2021 May 13;8(3):ENEURO.0089-21.2021. doi: 10.1523/ENEURO.0089-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33833046 Free PMC article.
References
-
- Riva P., Ratti A., and Venturin M. (2016). The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Current Alzheimer research 13, 1219–1231. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
