Stress granules: potential therapeutic targets for infectious and inflammatory diseases
- PMID: 37205103
- PMCID: PMC10185834
- DOI: 10.3389/fimmu.2023.1145346
Stress granules: potential therapeutic targets for infectious and inflammatory diseases
Abstract
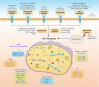
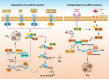
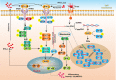
Eukaryotic cells are stimulated by external pressure such as that derived from heat shock, oxidative stress, nutrient deficiencies, or infections, which induce the formation of stress granules (SGs) that facilitates cellular adaptation to environmental pressures. As aggregated products of the translation initiation complex in the cytoplasm, SGs play important roles in cell gene expression and homeostasis. Infection induces SGs formation. Specifically, a pathogen that invades a host cell leverages the host cell translation machinery to complete the pathogen life cycle. In response, the host cell suspends translation, which leads to SGs formation, to resist pathogen invasion. This article reviews the production and function of SGs, the interaction between SGs and pathogens, and the relationship between SGs and pathogen-induced innate immunity to provide directions for further research into anti-infection and anti-inflammatory disease strategies.
Keywords: infection; inflammation; innate immunity; stress granules; therapeutic targets.
Copyright © 2023 Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Stress granules: regulators or by-products?FEBS J. 2022 Jan;289(2):363-373. doi: 10.1111/febs.15821. Epub 2021 Mar 27. FEBS J. 2022. PMID: 33725420
-
Defining the Role of Stress Granules in Innate Immune Suppression by the Herpes Simplex Virus 1 Endoribonuclease VHS.J Virol. 2018 Jul 17;92(15):e00829-18. doi: 10.1128/JVI.00829-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793959 Free PMC article.
-
Newcastle disease virus induces stable formation of bona fide stress granules to facilitate viral replication through manipulating host protein translation.FASEB J. 2017 Apr;31(4):1337-1353. doi: 10.1096/fj.201600980R. Epub 2016 Dec 23. FASEB J. 2017. PMID: 28011649
-
Role of stress granules in tumorigenesis and cancer therapy.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):189006. doi: 10.1016/j.bbcan.2023.189006. Epub 2023 Oct 31. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37913942 Review.
-
Heat shock proteins-driven stress granule dynamics: yet another avenue for cell survival.Apoptosis. 2021 Aug;26(7-8):371-384. doi: 10.1007/s10495-021-01678-w. Epub 2021 May 12. Apoptosis. 2021. PMID: 33978921 Review.
Cited by
-
Formation of the NLRP3 inflammasome inhibits stress granule assembly by multiple mechanisms.J Biochem. 2024 May 31;175(6):629-641. doi: 10.1093/jb/mvae009. J Biochem. 2024. PMID: 38299728 Free PMC article.
-
Metal-polyphenol-network coated R612F nanoparticles reduce drug resistance in hepatocellular carcinoma by inhibiting stress granules.Cell Death Discov. 2024 Aug 28;10(1):384. doi: 10.1038/s41420-024-02161-6. Cell Death Discov. 2024. PMID: 39198406 Free PMC article.
-
Fragile X Messenger Ribonucleoprotein Protein and Its Multifunctionality: From Cytosol to Nucleolus and Back.Biomolecules. 2024 Mar 26;14(4):399. doi: 10.3390/biom14040399. Biomolecules. 2024. PMID: 38672417 Free PMC article. Review.
-
Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system.World J Hepatol. 2024 Feb 27;16(2):251-263. doi: 10.4254/wjh.v16.i2.251. World J Hepatol. 2024. PMID: 38495274 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

