Development of Novel High-Affinity Antagonists for the Relaxin Family Peptide Receptor 1
- PMID: 37200817
- PMCID: PMC10186362
- DOI: 10.1021/acsptsci.3c00053
Development of Novel High-Affinity Antagonists for the Relaxin Family Peptide Receptor 1
Abstract
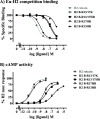
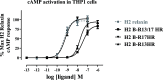
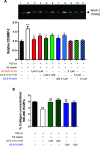
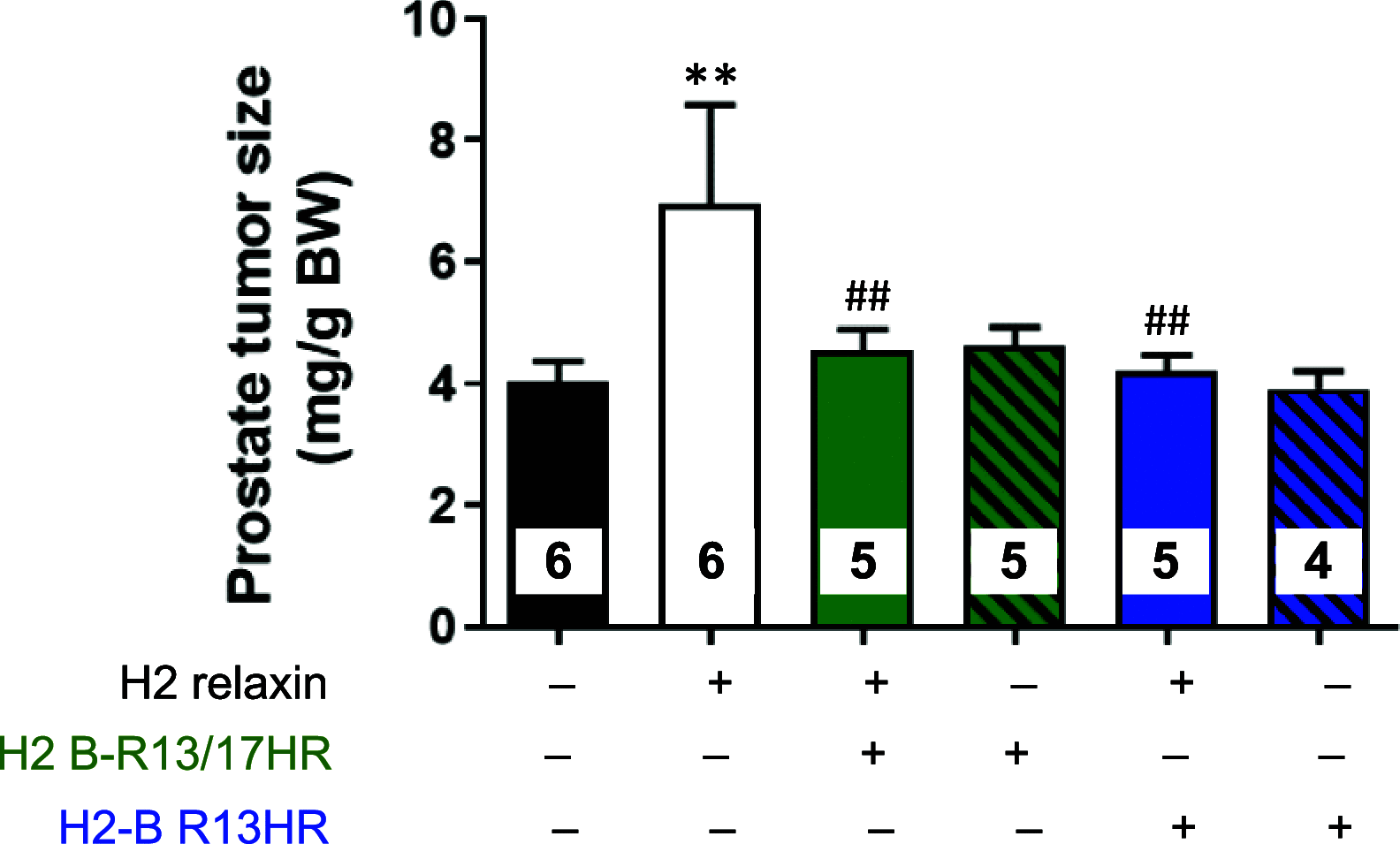
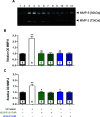
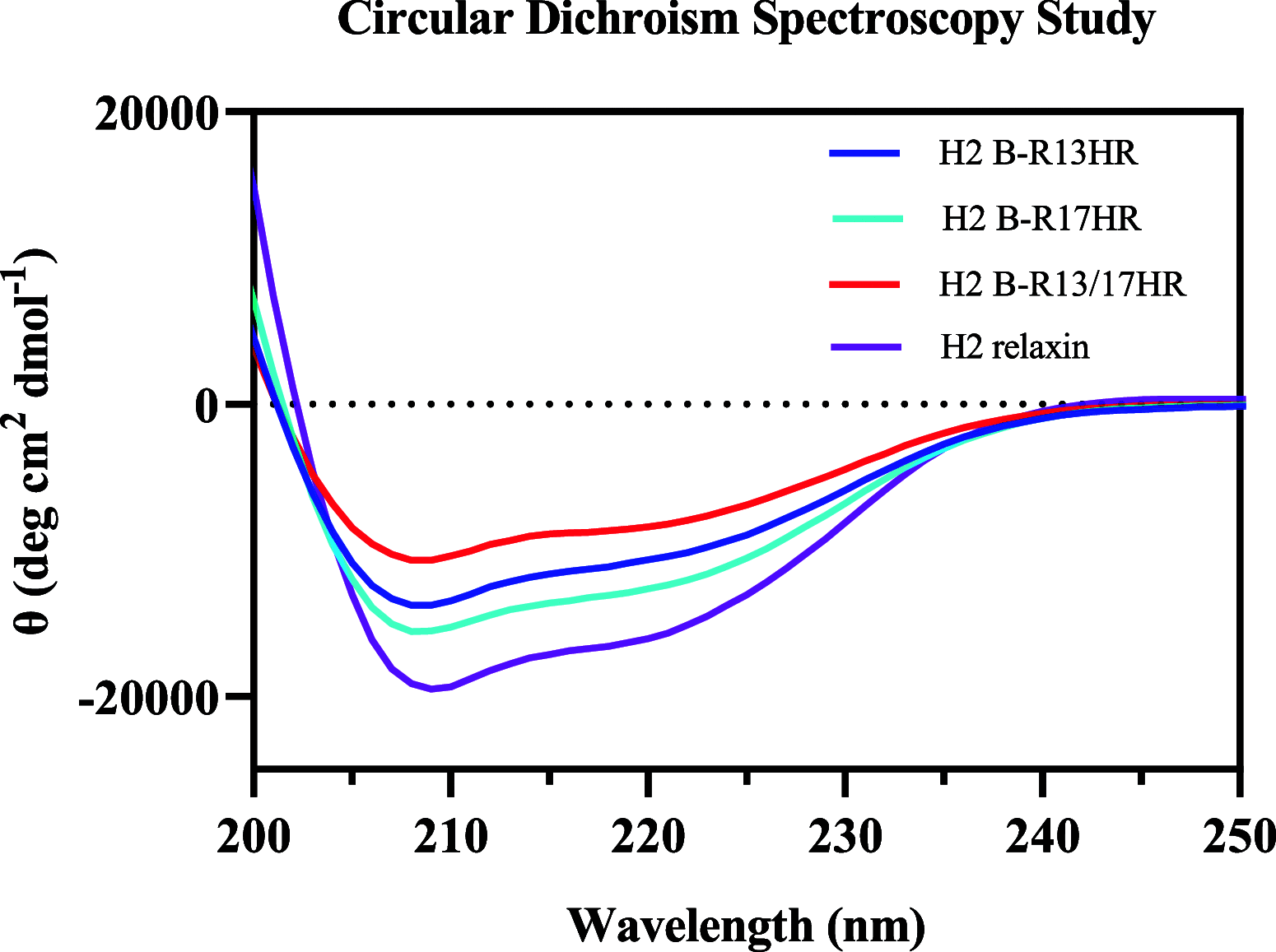
H2 relaxin is a peptide hormone that exerts its biological actions through the G protein-coupled receptor, RXFP1. The numerous important biological functions of H2 relaxin, including potent renal, vasodilatory, cardioprotective, and anti-fibrotic actions, have resulted in considerable interest in its use as a therapeutic for various cardiovascular diseases and other fibrotic indications. Interestingly though, H2 relaxin and RXFP1 have been shown to be overexpressed in prostate cancer, allowing for the downregulation or blocking of relaxin/RXFP1 to decrease prostate tumor growth. These findings suggest the application of an RXFP1 antagonist for the treatment of prostate cancer. However, these therapeutically relevant actions are still poorly understood and have been hindered by the lack of a high-affinity antagonist. In this study, we chemically synthesized three novel H2 relaxin analogues that have complex insulin-like structures with two chains (A and B) and three disulfide bridges. We report here the structure-activity relationship studies on H2 relaxin that resulted in the development of a novel high-affinity RXFP1 antagonist, H2 B-R13HR (∼40 nM), that has only one extra methylene group in the side chain of arginine 13 in the B-chain (ArgB13) of H2 relaxin. Most notably, the synthetic peptide was shown to be active in a mouse model of prostate tumor growth in vivo where it inhibited relaxin-mediated tumor growth. Our compound H2 B-R13HR will be an important research tool to understand relaxin actions through RXFP1 and may be a potential lead compound for the treatment of prostate cancer.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The chemically synthesized human relaxin-2 analog, B-R13/17K H2, is an RXFP1 antagonist.Amino Acids. 2010 Jul;39(2):409-16. doi: 10.1007/s00726-009-0454-1. Epub 2009 Dec 31. Amino Acids. 2010. PMID: 20043231
-
A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1.Chem Sci. 2016 Jun 1;7(6):3805-3819. doi: 10.1039/c5sc04754d. Epub 2016 Feb 26. Chem Sci. 2016. PMID: 30155023 Free PMC article.
-
Further Developments towards a Minimal Potent Derivative of Human Relaxin-2.Int J Mol Sci. 2023 Aug 11;24(16):12670. doi: 10.3390/ijms241612670. Int J Mol Sci. 2023. PMID: 37628851 Free PMC article.
-
The roles of the A- and B-chains of human relaxin-2 and -3 on their biological activity.Curr Protein Pept Sci. 2010 Dec;11(8):719-24. doi: 10.2174/138920310794557736. Curr Protein Pept Sci. 2010. PMID: 21235507 Review.
-
Understanding relaxin signalling at the cellular level.Mol Cell Endocrinol. 2019 May 1;487:24-33. doi: 10.1016/j.mce.2018.12.017. Epub 2018 Dec 25. Mol Cell Endocrinol. 2019. PMID: 30592984 Review.
Cited by
-
RNA nanotherapeutics with fibrosis overexpression and retention for MASH treatment.Nat Commun. 2024 Aug 27;15(1):7263. doi: 10.1038/s41467-024-51571-8. Nat Commun. 2024. PMID: 39191801 Free PMC article.
-
Significance of plasma relaxin-2 levels in patients with primary hypertension and type 2 diabetes mellitus.Wien Med Wochenschr. 2024 May;174(7-8):161-172. doi: 10.1007/s10354-024-01035-x. Epub 2024 Mar 7. Wien Med Wochenschr. 2024. PMID: 38451351 English.
References
-
- Bathgate R. A.; Hsueh A. J.; Sherwood O. D.. Physiology and Molecular Biology of the Relaxin Peptide Family. In Physiology of Reproduction, 3rd ed.; Neill J. D., Ed. Elsevier: San Diego, 2006; pp. 679–770, 10.1016/B978-012515400-0/50021-X - DOI
LinkOut - more resources
Full Text Sources
