Clinical efficacy and safety of interleukin-1 blockade in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials
- PMID: 37199379
- PMCID: PMC10198008
- DOI: 10.1080/07853890.2023.2208872
Clinical efficacy and safety of interleukin-1 blockade in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials
Abstract
Objective: This study evaluated the clinical efficacy and safety of interleukin-1 (IL-1) blockade for patients with COVID-19.
Methods: The PubMed, Web of Science, Ovid Medline, Embase and Cochrane Library databases were searched for relevant articles from their inception to 25 September 2022. Only randomized clinical trials (RCTs) that assessed the clinical efficacy and safety of IL-1 blockade in the treatment of patients with COVID-19 were included.
Results: This meta-analysis included seven RCTs. No significant difference in the all-cause mortality rate of patients with COVID-19 was observed between the IL-1 blockade and control groups (7.7 vs. 10.5%, odds ratio [OR] = 0.83, 95% confidence interval [CI] 0.57-1.22; I2 = 18%). However, the study group was at significantly lower risk of requiring mechanical ventilation (MV) compared with the control group (OR = 0.53, 95% CI 0.32-0.86; I2 = 24%). Finally, the risk of adverse events was similar between the two groups.
Conclusions: IL-1 blockade does not provide increased survival benefits in hospitalized patients with COVID-19, but it may reduce the need for MV. Furthermore, it is a safe agent for use in the treatment of COVID-19.>.
Keywords: Anakinra; COVID-19; SARS-CoV-2; canakinumab; interleukin-1.
Plain language summary
This systematic review and meta-analysis of randomized clinical trials (RCTs) evaluated the clinical efficacy and safety of interleukin-1 (IL-1) blockade for patients with COVID-19.Based on the analysis of six RCTs, no significant difference in the all-cause mortality rate of patients with COVID-19 was observed between the IL-1 blockade and control groups.The study group using IL1 was associated with a significantly lower risk of requiring mechanical ventilation compared with the control group.The risk of adverse events was similar between the study and the control groups.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



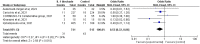

Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):468. doi: 10.1186/s13063-020-04453-5. Trials. 2020. PMID: 32493441 Free PMC article.
-
Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, first update.Clin Microbiol Infect. 2021 Aug;27(8):1076-1082. doi: 10.1016/j.cmi.2021.04.019. Epub 2021 Apr 27. Clin Microbiol Infect. 2021. PMID: 33915284 Free PMC article.
-
Efficacy and safety of azithromycin in Covid-19 patients: A systematic review and meta-analysis of randomized clinical trials.Rev Med Virol. 2022 Jan;32(1):e2258. doi: 10.1002/rmv.2258. Epub 2021 Jun 2. Rev Med Virol. 2022. PMID: 34077600 Free PMC article. Review.
-
Anakinra for the treatment of COVID-19 patients: a systematic review and meta-analysis.Eur J Med Res. 2023 Feb 25;28(1):100. doi: 10.1186/s40001-023-01072-z. Eur J Med Res. 2023. PMID: 36841793 Free PMC article. Review.
Cited by
-
NLRP3 Inflammasomes: Dual Function in Infectious Diseases.J Immunol. 2024 Aug 15;213(4):407-417. doi: 10.4049/jimmunol.2300745. J Immunol. 2024. PMID: 39102612 Free PMC article. Review.
-
NLRP3 Inflammasome Involvement in Heart, Liver, and Lung Diseases-A Lesson from Cytokine Storm Syndrome.Int J Mol Sci. 2023 Nov 21;24(23):16556. doi: 10.3390/ijms242316556. Int J Mol Sci. 2023. PMID: 38068879 Free PMC article. Review.
References
-
- WHO; 2022. [cited 2022 Sep 24]. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.Who.Int/.
-
- Berlin DA, Gulick RM, Martinez FJ.. Severe COVID-19. N Engl J Med. 2020;383(25):2451–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
