AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma
- PMID: 37199193
- PMCID: PMC10663390
- DOI: 10.1097/HEP.0000000000000466
AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma
Erratum in
-
Erratum: AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma.Hepatology. 2023 Dec 1;78(6):E105. doi: 10.1097/HEP.0000000000000621. Epub 2023 Oct 16. Hepatology. 2023. PMID: 37843497 No abstract available.
Conflict of interest statement
Amit G. Singal consults for Genentech, AstraZeneca, Eisai, Bayer, Boston Scientific, Exelixis, FujiFilm Medical Sciences, Exact Sciences, Glycotest, and Universal Diagnostics. Joseph M. Llovet consults for and received grants from Eisai, Bayer, and Ipsen. He consults for Merck, Bristol-Myers Squibb, Eli Lilly, Roche, Genentech, Glycotest, Boston Scientific, Exelixis, Bluejay, AstraZeneca, Omega Therapeutics, Mina Alpha, and Captor. Mark Yarchoan consults for and received institutional grants from Genentech. He own stock in and has other interests in Adventris Pharmaceuticals. He consults for Exelixis, Eisai, AstraZeneca, Geneos, Replimune, and Hepion. He received institutional grants from Incyte and Bristol-Myers Squibb. Laura A. Dawson received institutional grants from Merck. Janice H. Jou received grants from Gilead. Laura M. Kulik consults for, advises and is on the speakers’ bureau for Eisai. She consults for and advises Eisai, Exelixis, Genetec, and AstraZeneca. She advises and is on the speakers’ bureau for Gilead. She consults for Merck and Bayer. She advises Hepion and Fujifilm. She received grants from HCC Target and Glycotest. Vatche G. Agopian received grants from Early Diagnostics. Jorge A. Marrero consults for Glycotest and AstraZeneca. Daniel B. Brown received institutional grants from Sirtex Medical and Guerbet. William S. Rilling consults for and advises Boston Scientific. He consults for Varian, Terumo, BD Bard, and AstraZeneca. Lipika Goyal advises and or consults for Alentis Therapeutics, Black Diamond, Exelixis, Genentech, H3Biomedicine, Kinnate, Incyte Corporation, QED Therapeutics, Merck, Servier, Sirtex Medical, Surface Oncology, Taiho Oncology, TranstheraBio, and Tyra Biosciences. She is on the DSMC for AstraZeneca. Alice C. Wei is on the speakers’ bureau for AstraZeneca. She consults for Histosonics and Biosapien. The remaining authors have no conflicts to report.
Figures




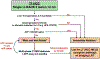


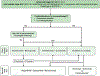
Similar articles
-
Quality measures in HCC care by the Practice Metrics Committee of the American Association for the Study of Liver Diseases.Hepatology. 2022 May;75(5):1289-1299. doi: 10.1002/hep.32240. Epub 2021 Dec 20. Hepatology. 2022. PMID: 34778999
-
A flexible three-dimensional heterophase computed tomography hepatocellular carcinoma detection algorithm for generalizable and practical screening.Hepatol Commun. 2022 Oct;6(10):2901-2913. doi: 10.1002/hep4.2029. Epub 2022 Jul 19. Hepatol Commun. 2022. PMID: 35852311 Free PMC article.
-
Quantitative dual-energy computed tomography with cesium as a novel contrast agent for localization of thermochemical ablation in phantoms and ex vivo models.Med Phys. 2023 Dec;50(12):7879-7890. doi: 10.1002/mp.16558. Epub 2023 Jul 6. Med Phys. 2023. PMID: 37409792 Free PMC article.
-
Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update.Clin Mol Hepatol. 2019 Sep;25(3):245-263. doi: 10.3350/cmh.2018.0090. Epub 2019 Feb 14. Clin Mol Hepatol. 2019. PMID: 30759967 Free PMC article. Review.
-
The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022.ESMO Open. 2023 Jun;8(3):101567. doi: 10.1016/j.esmoop.2023.101567. Epub 2023 May 31. ESMO Open. 2023. PMID: 37263081 Free PMC article. Review.
Cited by
-
National Liver Cancer Screening Trial (TRACER) study protocol.Hepatol Commun. 2024 Nov 4;8(11):e0565. doi: 10.1097/HC9.0000000000000565. eCollection 2024 Nov 1. Hepatol Commun. 2024. PMID: 39495136 Free PMC article.
-
Efficacy and safety of regorafenib as a first-line agent alone or in combination with an immune checkpoint inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study.J Gastrointest Oncol. 2024 Jun 30;15(3):1072-1081. doi: 10.21037/jgo-24-315. Epub 2024 Jun 27. J Gastrointest Oncol. 2024. PMID: 38989425 Free PMC article.
-
Therapeutic Approach to Post-Transplant Recurrence of Hepatocellular Carcinoma: Certainties and Open Issues.Cancers (Basel). 2023 Nov 26;15(23):5593. doi: 10.3390/cancers15235593. Cancers (Basel). 2023. PMID: 38067299 Free PMC article. Review.
-
Can Dynamic Contrast-Enhanced MRI Be Used to Differentiate Hepatic Hemangioma from Other Lesions in Early Infancy?Indian J Radiol Imaging. 2024 Apr 21;34(4):612-619. doi: 10.1055/s-0044-1785208. eCollection 2024 Oct. Indian J Radiol Imaging. 2024. PMID: 39318559 Free PMC article.
-
Inter-reader agreement for LR-M imaging features: a premise for better imaging-based diagnosis in liver imaging.J Liver Cancer. 2024 Sep;24(2):124-125. doi: 10.17998/jlc.2024.08.06. Epub 2024 Aug 13. J Liver Cancer. 2024. PMID: 39134467 Free PMC article. No abstract available.
References
-
- OCEBM Levels of Evidence Working Group: Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-e...
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

