Cooperation between Prostaglandin E2 and Epidermal Growth Factor Receptor in Cancer Progression: A Dual Target for Cancer Therapy
- PMID: 37190301
- PMCID: PMC10136831
- DOI: 10.3390/cancers15082374
Cooperation between Prostaglandin E2 and Epidermal Growth Factor Receptor in Cancer Progression: A Dual Target for Cancer Therapy
Abstract
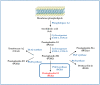
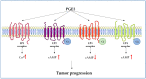
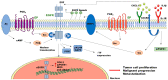
It is recognized that prostaglandin E2 (PGE2) is one key lipid mediator involved in chronic inflammation, and it is directly implicated in tumor development by regulating cancer cell growth and migration, apoptosis, epithelial-mesenchymal transition, angiogenesis, and immune escape. In addition, the expression of the enzymes involved in PGE2 synthesis, cyclooxygenase 2 (COX-2) and microsomal prostaglandin E synthase 1 (mPGES1), positively correlates with tumor progression and aggressiveness, clearly indicating the crucial role of the entire pathway in cancer. Moreover, several lines of evidence suggest that the COX2/mPGES1/PGE2 inflammatory axis is involved in the modulation of epidermal growth factor receptor (EGFR) signaling to reinforce the oncogenic drive of EGFR activation. Similarly, EGFR activation promotes the induction of COX2/mPGES1 expression and PGE2 production. In this review, we describe the interplay between COX2/mPGES1/PGE2 and EGFR in cancer, and new therapeutic strategies that target this signaling pathway, to outline the importance of the modulation of the inflammatory process in cancer fighting.
Keywords: COX2; EGFR; PGE2; TKRs; cancer; extrinsic inflammation; intrinsic inflammation; mPGES1; tumor angiogenesis; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Upregulation of C-C chemokine receptor type 7 expression by membrane-associated prostaglandin E synthase-1/prostaglandin E2 requires glycogen synthase kinase 3β-mediated signal transduction in colon cancer cells.Mol Med Rep. 2015 Nov;12(5):7169-75. doi: 10.3892/mmr.2015.4290. Epub 2015 Sep 3. Mol Med Rep. 2015. PMID: 26352871
-
COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):1117-1122. doi: 10.1073/pnas.1612920114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096371 Free PMC article.
-
Kruppel-like factor 5 transcription factor promotes microsomal prostaglandin E2 synthase 1 gene transcription in breast cancer.J Biol Chem. 2013 Sep 13;288(37):26731-40. doi: 10.1074/jbc.M113.483958. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913682 Free PMC article.
-
Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity.Biology (Basel). 2020 Dec 1;9(12):434. doi: 10.3390/biology9120434. Biology (Basel). 2020. PMID: 33271839 Free PMC article. Review.
-
EP4 receptor as a novel promising therapeutic target in colon cancer.Pathol Res Pract. 2020 Dec;216(12):153247. doi: 10.1016/j.prp.2020.153247. Epub 2020 Oct 19. Pathol Res Pract. 2020. PMID: 33190014 Review.
Cited by
-
Exploring the Mechanisms of Traditional Chinese Herbal Therapy in Gastric Cancer: A Comprehensive Network Pharmacology Study of the Tiao-Yuan-Tong-Wei decoction.Pharmaceuticals (Basel). 2024 Mar 25;17(4):414. doi: 10.3390/ph17040414. Pharmaceuticals (Basel). 2024. PMID: 38675376 Free PMC article.
-
Preoperative Serum Levels of PDGF-AB, PDGF-BB, TGF-α, EGF and ANG-2 in the Diagnosis of Endometrial Cancer.Cancers (Basel). 2023 Sep 30;15(19):4815. doi: 10.3390/cancers15194815. Cancers (Basel). 2023. PMID: 37835508 Free PMC article.
-
Amphiregulin orchestrates the paracrine immune-suppressive function of amniotic-derived cells through its interplay with COX-2/PGE2/EP4 axis.iScience. 2024 Jul 14;27(8):110508. doi: 10.1016/j.isci.2024.110508. eCollection 2024 Aug 16. iScience. 2024. PMID: 39156643 Free PMC article.
-
A novel bioresponsive self-immolative spacer based on aza-quinone methide reactivity for the controlled release of thiols, phenols, amines, sulfonamides or amides.Chem Sci. 2024 Apr 2;15(16):6168-6177. doi: 10.1039/d4sc01576b. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665538 Free PMC article.
-
Inflammation-related molecular signatures involved in the anticancer activities of brigatinib as well as the prognosis of EML4-ALK lung adenocarcinoma patient.Acta Pharmacol Sin. 2024 Jun;45(6):1252-1263. doi: 10.1038/s41401-024-01230-x. Epub 2024 Feb 15. Acta Pharmacol Sin. 2024. PMID: 38360931
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

