The neural pathway of the hyperthermic response to antagonists of the transient receptor potential vanilloid-1 channel
- PMID: 37187834
- PMCID: PMC10177699
- DOI: 10.1080/23328940.2023.2171671
The neural pathway of the hyperthermic response to antagonists of the transient receptor potential vanilloid-1 channel
Abstract
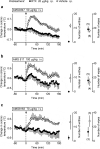
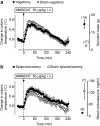
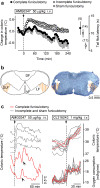
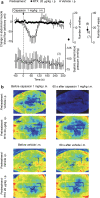
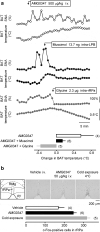
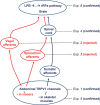
We identified the neural pathway of the hyperthermic response to TRPV1 antagonists. We showed that hyperthermia induced by i.v. AMG0347, AMG 517, or AMG8163 did not occur in rats with abdominal sensory nerves desensitized by pretreatment with a low i.p. dose of resiniferatoxin (RTX, TRPV1 agonist). However, neither bilateral vagotomy nor bilateral transection of the greater splanchnic nerve attenuated AMG0347-induced hyperthermia. Yet, this hyperthermia was attenuated by bilateral high cervical transection of the spinal dorsolateral funiculus (DLF). To explain the extra-splanchnic, spinal mediation of TRPV1 antagonist-induced hyperthermia, we proposed that abdominal signals that drive this hyperthermia originate in skeletal muscles - not viscera. If so, in order to prevent TRPV1 antagonist-induced hyperthermia, the desensitization caused by i.p. RTX should spread into the abdominal-wall muscles. Indeed, we found that the local hypoperfusion response to capsaicin (TRPV1 agonist) in the abdominal-wall muscles was absent in i.p. RTX-desensitized rats. We then showed that the most upstream (lateral parabrachial, LPB) and the most downstream (rostral raphe pallidus) nuclei of the intrabrain pathway that controls autonomic cold defenses are also required for the hyperthermic response to i.v. AMG0347. Injection of muscimol (inhibitor of neuronal activity) into the LPB or injection of glycine (inhibitory neurotransmitter) into the raphe blocked the hyperthermic response to i.v. AMG0347, whereas i.v. AMG0347 increased the number of c-Fos cells in the raphe. We conclude that the neural pathway of TRPV1 antagonist-induced hyperthermia involves TRPV1-expressing sensory nerves in trunk muscles, the DLF, and the same LPB-raphe pathway that controls autonomic cold defenses.
Keywords: TRPV1 blockers; Thermoregulation; hyperthermia; protons; skeletal muscle; spinal cord; splanchnic; vagus.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
A.A.R. is an officer and director of Catalina Pharma, Inc. and Zharko Pharma, Inc.; he has consulted for TRPV1 programs at several pharmaceutical companies, and his laboratory conducted paid research on TRPV1 for Amgen Inc., Abbott Laboratories, and AbbVie Inc.
Figures
Similar articles
-
Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors.J Neurosci. 2007 Jul 11;27(28):7459-68. doi: 10.1523/JNEUROSCI.1483-07.2007. J Neurosci. 2007. PMID: 17626206 Free PMC article.
-
Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis.Pharmacol Ther. 2020 Apr;208:107474. doi: 10.1016/j.pharmthera.2020.107474. Epub 2020 Jan 9. Pharmacol Ther. 2020. PMID: 31926897 Review.
-
Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade.J Pharmacol Exp Ther. 2007 Oct;323(1):128-37. doi: 10.1124/jpet.107.125674. Epub 2007 Jul 25. J Pharmacol Exp Ther. 2007. PMID: 17652633
-
TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds' pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development.Acta Physiol (Oxf). 2018 Jul;223(3):e13038. doi: 10.1111/apha.13038. Epub 2018 Feb 16. Acta Physiol (Oxf). 2018. PMID: 29352512 Free PMC article.
-
The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not.Pharmacol Rev. 2009 Sep;61(3):228-61. doi: 10.1124/pr.109.001263. Epub 2009 Sep 11. Pharmacol Rev. 2009. PMID: 19749171 Free PMC article. Review.
Cited by
-
TRPV1 Channels in the Central Nervous System as Drug Targets.Pharmaceuticals (Basel). 2024 Jun 7;17(6):756. doi: 10.3390/ph17060756. Pharmaceuticals (Basel). 2024. PMID: 38931423 Free PMC article. Review.
-
Acid-sensing ion channel 1a modulation of apoptosis in acidosis-related diseases: implications for therapeutic intervention.Cell Death Discov. 2023 Sep 4;9(1):330. doi: 10.1038/s41420-023-01624-6. Cell Death Discov. 2023. PMID: 37666823 Free PMC article. Review.
-
From capsaicin to TRPV1: The "hot" legacy of János Szolcsányi.Temperature (Austin). 2023 May 11;10(1):1-2. doi: 10.1080/23328940.2023.2194191. eCollection 2023. Temperature (Austin). 2023. PMID: 37187831 Free PMC article. No abstract available.
-
Selection of preferred thermal environment and cold-avoidance responses in rats rely on signals transduced by the dorsal portion of the lateral funiculus of the spinal cord.Temperature (Austin). 2023 Apr 7;10(1):121-135. doi: 10.1080/23328940.2023.2191378. eCollection 2023. Temperature (Austin). 2023. PMID: 37187830 Free PMC article.
References
-
- Garami A, Shimansky YP, Rumbus Z, et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: insights from mathematical modeling and meta-analysis. Pharmacol Ther. 2020;208:107474. - PubMed
-
- Gavva NR, Treanor JJ, Garami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–210. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
