Distinct profiles of oxylipid mediators in liver, lung, and placenta after maternal nano-TiO2 nanoparticle inhalation exposure
- PMID: 37181648
- PMCID: PMC10167894
- DOI: 10.1039/d2va00300g
Distinct profiles of oxylipid mediators in liver, lung, and placenta after maternal nano-TiO2 nanoparticle inhalation exposure
Abstract
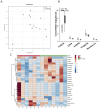
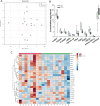
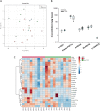
Nano-titanium dioxide (nano-TiO2) is a widely used nanomaterial found in several industrial and consumer products, including surface coatings, paints, sunscreens and cosmetics, among others. Studies have linked gestational exposure to nano-TiO2 with negative maternal and fetal health outcomes. For example, maternal pulmonary exposure to nano-TiO2 during gestation has been associated not only with maternal, but also fetal microvascular dysfunction in a rat model. One mediator of this altered vascular reactivity and inflammation is oxylipid signaling. Oxylipids are formed from dietary lipids through several enzyme-controlled pathways as well as through oxidation by reactive oxygen species. Oxylipids have been linked to control of vascular tone, inflammation, pain and other physiological and disease processes. In this study, we use a sensitive UPLC-MS/MS based analysis to probe the global oxylipid response in liver, lung, and placenta of pregnant rats exposed to nano-TiO2 aerosols. Each organ presented distinct patterns in oxylipid signaling, as assessed by principal component and hierarchical clustering heatmap analysis. In general, pro-inflammatory mediators, such as 5-hydroxyeicosatetraenoic acid (1.6 fold change) were elevated in the liver, while in the lung, anti-inflammatory and pro-resolving mediators such as 17-hydroxy docosahexaenoic acid (1.4 fold change) were elevated. In the placenta the levels of oxylipid mediators were generally decreased, both inflammatory (e.g. PGE2, 0.52 fold change) and anti-inflammatory (e.g. Leukotriene B4, 0.49 fold change). This study, the first to quantitate the levels of these oxylipids simultaneously after nano-TiO2 exposure, shows the complex interplay of pro- and anti-inflammatory mediators from multiple lipid classes and highlights the limitations of monitoring the levels of oxylipid mediators in isolation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Nano-titanium dioxide inhalation exposure during gestation drives redox dysregulation and vascular dysfunction across generations.Part Fibre Toxicol. 2022 Mar 9;19(1):18. doi: 10.1186/s12989-022-00457-y. Part Fibre Toxicol. 2022. PMID: 35260159 Free PMC article.
-
Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics.Toxicol Appl Pharmacol. 2019 Mar 15;367:51-61. doi: 10.1016/j.taap.2019.01.024. Epub 2019 Feb 1. Toxicol Appl Pharmacol. 2019. PMID: 30711534 Free PMC article.
-
Nanomaterial Inhalation During Pregnancy Alters Systemic Vascular Function in a Cyclooxygenase-Dependent Manner.Toxicol Sci. 2022 Jul 28;188(2):219-233. doi: 10.1093/toxsci/kfac055. Toxicol Sci. 2022. PMID: 35642938 Free PMC article.
-
Safety of titanium dioxide nanoparticles in cosmetics.J Eur Acad Dermatol Venereol. 2019 Nov;33 Suppl 7:34-46. doi: 10.1111/jdv.15943. J Eur Acad Dermatol Venereol. 2019. PMID: 31588611 Review.
-
Murine liver damage caused by exposure to nano-titanium dioxide.Nanotechnology. 2016 Mar 18;27(11):112001. doi: 10.1088/0957-4484/27/11/112001. Epub 2016 Feb 12. Nanotechnology. 2016. PMID: 26871200 Review.
Cited by
-
Guidelines for assessing maternal cardiovascular physiology during pregnancy and postpartum.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H191-H220. doi: 10.1152/ajpheart.00055.2024. Epub 2024 May 17. Am J Physiol Heart Circ Physiol. 2024. PMID: 38758127 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
