The use of systemic corticosteroids in asthma management in Latin American countries
- PMID: 37179538
- PMCID: PMC10172569
- DOI: 10.1016/j.waojou.2023.100760
The use of systemic corticosteroids in asthma management in Latin American countries
Abstract
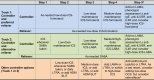
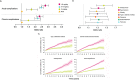
The stepwise treatment approach recommended by the Global Initiative for Asthma (GINA) includes systemic corticosteroids (SCS) suggested as a final step if asthma is severe and/or difficult to treat. Yet, despite the effectiveness of SCS, they are also associated with potentially irreversible adverse outcomes such as type 2 diabetes, adrenal suppression, and cardiovascular disease. Based on recent data indicating that the risk of developing these conditions can increase after as few as 4 short-term (burst) courses of SCS, even patients with mild asthma who receive SCS occasionally for exacerbations are also at risk of these events. As a result, recent updates by GINA and the Latin American Thoracic Society recommend decreasing SCS use by optimizing administration of non-SCS therapies and/or increasing the use of alternatives, such as biologic agents. Recent and ongoing studies characterizing treatment patterns among patients with asthma have revealed alarming trends suggesting the widespread overuse of SCS around the world. In Latin America, asthma prevalence is approximately 17%, and data suggest that the majority of patients have uncontrolled disease. In this review, we summarize currently available data on asthma treatment patterns in Latin America, which indicate that SCS are prescribed to 20-40% of patients with asthma considered to be well controlled and over 50% of patients with uncontrolled disease. We also offer potential strategies to help reduce SCS use for asthma in everyday clinical practice.
Keywords: Adverse effects; Biological products; Latin America; Severe asthma; Systemic corticosteroids.
© 2023 The Authors.
Figures
Similar articles
-
Systemic corticosteroids in asthma: A call to action from World Allergy Organization and Respiratory Effectiveness Group.World Allergy Organ J. 2022 Dec 10;15(12):100726. doi: 10.1016/j.waojou.2022.100726. eCollection 2022 Dec. World Allergy Organ J. 2022. PMID: 36582404 Free PMC article. Review.
-
The burden of systemic corticosteroid use in asthma management in Asia.Respirology. 2023 Aug;28(8):744-757. doi: 10.1111/resp.14533. Epub 2023 Jun 10. Respirology. 2023. PMID: 37301540 Review.
-
Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety.Eur Respir Rev. 2020 Apr 3;29(155):190151. doi: 10.1183/16000617.0151-2019. Print 2020 Mar 31. Eur Respir Rev. 2020. PMID: 32245768 Free PMC article. Review.
-
[Latin-American Consensus on Difficult-to-Control Asthma. 2008 Update].Drugs Today (Barc). 2008 Jun;44 Suppl 3:1-43. Drugs Today (Barc). 2008. PMID: 19093041 Review. Spanish.
-
It is time to change the way we manage mild asthma: an update in GINA 2019.Respir Res. 2019 Aug 14;20(1):183. doi: 10.1186/s12931-019-1159-y. Respir Res. 2019. PMID: 31412856 Free PMC article.
Cited by
-
Oral sub-chronic toxicity of fingerroot (Boesenbergia rotunda) rhizome extract formulation in Wistar rats.Toxicol Rep. 2024 Jan 30;12:224-233. doi: 10.1016/j.toxrep.2024.01.013. eCollection 2024 Jun. Toxicol Rep. 2024. PMID: 38328737 Free PMC article.
-
Challenges and Opportunities in COPD Management in Latin America: A Review of Inhalation Therapies and Advanced Drug Delivery Systems.Pharmaceutics. 2024 Oct 11;16(10):1318. doi: 10.3390/pharmaceutics16101318. Pharmaceutics. 2024. PMID: 39458647 Free PMC article. Review.
-
Real-World Effectiveness of Mepolizumab in Severe Asthma: Results from the Multi-country, Self-controlled Nucala Effectiveness Study (NEST).Adv Ther. 2024 Nov;41(11):4008-4031. doi: 10.1007/s12325-024-02967-x. Epub 2024 Aug 31. Adv Ther. 2024. PMID: 39215767 Free PMC article.
References
-
- GBD Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. - PMC - PubMed
-
- Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. - PubMed
-
- Hankin C.S., Bronstone A., Wang Z., Small M.B., Buck P. Estimated prevalence and economic burden of severe, uncontrolled asthma in the United States. J Allergy Clin Immunol. 2013;131:AB126.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

