Rivastigmine-Benzimidazole Hybrids as Promising Multitarget Metal-Modulating Compounds for Potential Treatment of Neurodegenerative Diseases
- PMID: 37176018
- PMCID: PMC10179505
- DOI: 10.3390/ijms24098312
Rivastigmine-Benzimidazole Hybrids as Promising Multitarget Metal-Modulating Compounds for Potential Treatment of Neurodegenerative Diseases
Abstract
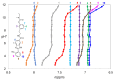
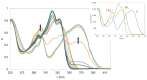
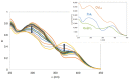
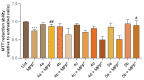
With the goal of combating the multi-faceted Alzheimer's disease (AD), a series of Rivastigmine-Benzimidazole (RIV-BIM) hybrids was recently reported by us as multitarget-directed ligands, thanks to their capacity to tackle important hallmarks of AD. In particular, they exhibited antioxidant activity, acted as cholinesterase inhibitors, and inhibited amyloid-β (Aβ) aggregation. Herein, we moved forward in this project, studying their ability to chelate redox-active biometal ions, Cu(II) and Fe(III), with widely recognized roles in the generation of oxidative reactive species and in protein misfolding and aggregation in both AD and Parkinson's disease (PD). Although Cu(II) chelation showed higher efficiency for the positional isomers of series 5 than those of series 4 of the hybrids, the Aβ-aggregation inhibition appears more dependent on their capacity for fibril intercalation than on copper chelation. Since monoamine oxidases (MAOs) are also important targets for the treatment of AD and PD, the capacity of these hybrids to inhibit MAO-A and MAO-B was evaluated, and they showed higher activity and selectivity for MAO-A. The rationalization of the experimental evaluations (metal chelation and MAO inhibition) was supported by computational molecular modeling studies. Finally, some compounds showed also neuroprotective effects in human neuroblastoma (SH-SY5Y cells) upon treatment with 1-methyl-4-phenylpyridinium (MPP+), a neurotoxic metabolite of a Parkinsonian-inducing agent.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyloid-β aggregation; cholinesterases; metal chelation; monoamine oxidase; rivastigmine hybrids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
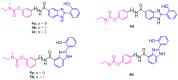


Similar articles
-
Exploiting the potential of rivastigmine-melatonin derivatives as multitarget metal-modulating drugs for neurodegenerative diseases.J Inorg Biochem. 2025 Jan;262:112734. doi: 10.1016/j.jinorgbio.2024.112734. Epub 2024 Sep 16. J Inorg Biochem. 2025. PMID: 39378762
-
Site-activated multi target iron chelators with acetylcholinesterase (AChE) and monoamine oxidase (MAO) inhibitory activities for Alzheimer's disease therapy.J Neural Transm (Vienna). 2022 Jun;129(5-6):715-721. doi: 10.1007/s00702-022-02462-z. Epub 2022 Feb 21. J Neural Transm (Vienna). 2022. PMID: 35190910
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
-
Multitarget-directed benzylideneindanone derivatives: anti-β-amyloid (Aβ) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer's disease.J Med Chem. 2012 Oct 11;55(19):8483-92. doi: 10.1021/jm300978h. Epub 2012 Oct 1. J Med Chem. 2012. PMID: 22978824
-
Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases.Mech Ageing Dev. 2005 Feb;126(2):317-26. doi: 10.1016/j.mad.2004.08.023. Mech Ageing Dev. 2005. PMID: 15621213 Review.
Cited by
-
Role of copper chelating agents: between old applications and new perspectives in neuroscience.Neural Regen Res. 2025 Mar 1;20(3):751-762. doi: 10.4103/NRR.NRR-D-24-00140. Epub 2024 May 13. Neural Regen Res. 2025. PMID: 38886940 Free PMC article.
-
Stepwise Structural Simplification of the Dihydroxyanthraquinone Moiety of a Multitarget Rhein-Based Anti-Alzheimer Lead to Improve Drug Metabolism and Pharmacokinetic Properties.Pharmaceutics. 2024 Jul 25;16(8):982. doi: 10.3390/pharmaceutics16080982. Pharmaceutics. 2024. PMID: 39204327 Free PMC article.
References
-
- Dorsey E.R., Elbaz A., Nichols E., Abbasi N., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
-
- Tricco A.C., Ashoor H.M., Soobiah C., Rios P., Veroniki A.A., Hamid J.S., Ivory J.D., Khan P.A., Yazdi F., Ghassemi M., et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: Systematic review and network metaanalysis. J. Am. Geriatr. Soc. 2018;66:170–178. doi: 10.1111/jgs.15069. - DOI - PubMed
-
- Hampel H., Mesulam M.M., Cuello A.C., Khachaturian A.S., Vergallo A., Farlow M.R., Snyder P.J., Giacobini E., Khachaturian Z.S. Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimers Dis. 2019;6:2–15. doi: 10.14283/jpad.2018.43. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

