Effects of Therapeutic Platelet-Rich Plasma on Overactive Bladder via Modulating Hyaluronan Synthesis in Ovariectomized Rat
- PMID: 37175945
- PMCID: PMC10179536
- DOI: 10.3390/ijms24098242
Effects of Therapeutic Platelet-Rich Plasma on Overactive Bladder via Modulating Hyaluronan Synthesis in Ovariectomized Rat
Abstract
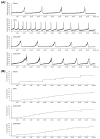
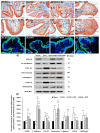
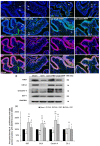
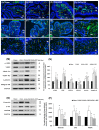
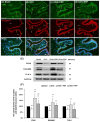
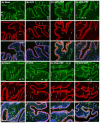
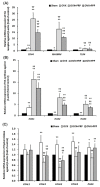
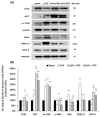
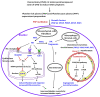
Postmenopausal women who have ovary hormone deficiency (OHD) may experience urological dysfunctions, such as overactive bladder (OAB) symptoms. This study used a female Sprague Dawley rat model that underwent bilateral ovariectomy (OVX) to simulate post-menopause in humans. The rats were treated with platelet-rich plasma (PRP) or platelet-poor plasma (PPP) after 12 months of OVX to investigate the therapeutic effects of PRP on OHD-induced OAB. The OVX-treated rats exhibited a decrease in the expression of urothelial barrier-associated proteins, altered hyaluronic acid (hyaluronan; HA) production, and exacerbated bladder pathological damage and interstitial fibrosis through NFƘB/COX-2 signaling pathways, which may contribute to OAB. In contrast, PRP instillation for four weeks regulated the inflammatory fibrotic biosynthesis, promoted cell proliferation and matrix synthesis of stroma, enhanced mucosal regeneration, and improved urothelial mucosa to alleviate OHD-induced bladder hyperactivity. PRP could release growth factors to promote angiogenic potential for bladder repair through laminin/integrin-α6 and VEGF/VEGF receptor signaling pathways in the pathogenesis of OHD-induced OAB. Furthermore, PRP enhanced the expression of HA receptors and hyaluronan synthases (HAS), reduced hyaluronidases (HYALs), modulated the fibroblast-myofibroblast transition, and increased angiogenesis and matrix synthesis via the PI3K/AKT/m-TOR pathway, resulting in bladder remodeling and regeneration.
Keywords: hyaluronan; ovariectomy; overactive bladder; platelet rich plasma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial.Int J Mol Sci. 2021 Aug 27;22(17):9296. doi: 10.3390/ijms22179296. Int J Mol Sci. 2021. PMID: 34502202 Free PMC article. Clinical Trial.
-
Therapeutic Effect of Platelet-Rich Plasma Improves Bladder Overactivity in the Pathogenesis of Ketamine-Induced Ulcerative Cystitis in a Rat Model.Int J Mol Sci. 2022 May 21;23(10):5771. doi: 10.3390/ijms23105771. Int J Mol Sci. 2022. PMID: 35628581 Free PMC article.
-
Elucidating Mechanisms of Bladder Repair after Hyaluronan Instillation in Ketamine-Induced Ulcerative Cystitis in Animal Model.Am J Pathol. 2017 Sep;187(9):1945-1959. doi: 10.1016/j.ajpath.2017.06.004. Am J Pathol. 2017. PMID: 28826558
-
An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: clinical and molecular aspects.Syst Biol Reprod Med. 2021 Jun;67(3):177-188. doi: 10.1080/19396368.2020.1862357. Epub 2021 Feb 25. Syst Biol Reprod Med. 2021. PMID: 33632047 Review.
-
Bladder Hyperactivity Induced by Oxidative Stress and Bladder Ischemia: A Review of Treatment Strategies with Antioxidants.Int J Mol Sci. 2021 Jun 2;22(11):6014. doi: 10.3390/ijms22116014. Int J Mol Sci. 2021. PMID: 34199527 Free PMC article. Review.
Cited by
-
Exploratory metabolomic analysis for characterizing the metabolic profile of the urinary bladder under estrogen deprivation.Front Endocrinol (Lausanne). 2024 May 31;15:1384115. doi: 10.3389/fendo.2024.1384115. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38883607 Free PMC article.
References
-
- Kuo H.-C. Videourodynamic precision diagnosis and treatment of lower urinary tract symptoms in women. Urol. Sci. 2021;32:94–101. doi: 10.4103/UROS.UROS_46_21. - DOI
-
- Haylen B.T., de Ridder D., Freeman R.M., Swift S.E., Berghmans B., Lee J., Monga A., Petri E., Rizk D.E., Sand P.K., et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- MOST109-2314-B-037-096/Ministry of Science and Technology of the People's Republic of China
- MOST111-2314-B-037-066/Ministry of Science and Technology of the People's Republic of China
- MOST111-2314-B-037-072/Ministry of Science and Technology of the People's Republic of China
- MOST 110-2222-E-020 -001/Ministry of Science and Technology of the People's Republic of China
- MOST 111-2221-E-020 -004/Ministry of Science and Technology of the People's Republic of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials

