Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation
- PMID: 37175896
- PMCID: PMC10179225
- DOI: 10.3390/ijms24098190
Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation
Abstract
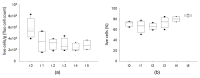
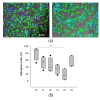
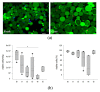
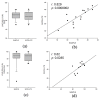
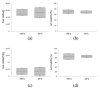
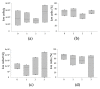
Adipose tissue (AT) is composed of a heterogeneous population which comprises both progenitor and differentiated cells. This heterogeneity allows a variety of roles for the AT, including regenerative functions. In fact, autologous AT is commonly used to repair soft tissue defects, and its cryopreservation could be a useful strategy to reduce the patient discomfort caused by multiple harvesting procedures. Our work aimed to characterize the cryopreserved AT and to validate its storage for up to three years for clinical applications. AT components (stromal vascular fraction-SVF and mature adipocytes) were isolated in fresh and cryopreserved samples using enzymatic digestion, and cell viability was assessed by immunofluorescence (IF) staining. Live, apoptotic and necrotic cells were quantified using cytometry by evaluating phosphatidylserine binding to fluorescent-labeled Annexin V. A multiparametric cytometry was also used to measure adipogenic (CD34+CD90+CD31-CD45-) and endothelial (CD34+CD31+CD45-) precursors and endothelial mature cells (CD34-CD31+CD45-). The maintenance of adipogenic abilities was evaluated using in vitro differentiation of SVF cultures and fluorescent lipid staining. We demonstrated that AT that is cryopreserved for up to three years maintains its differentiation potential and cellular composition. Given our results, a clinical study was started, and two patients had successful transplants without any complications using autologous cryopreserved AT.
Keywords: adipocytes; adipose tissue; autologous transplantation; regenerative medicine; stromal vascular fraction; tissue banking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media.Stem Cell Res Ther. 2018 May 11;9(1):130. doi: 10.1186/s13287-018-0886-1. Stem Cell Res Ther. 2018. PMID: 29751821 Free PMC article.
-
Effect of Cryopreservation on Human Adipose Tissue and Isolated Stromal Vascular Fraction Cells: In Vitro and In Vivo Analyses.Plast Reconstr Surg. 2018 Feb;141(2):232e-243e. doi: 10.1097/PRS.0000000000004030. Plast Reconstr Surg. 2018. PMID: 29369990
-
Adipogenic potential of adipose stem cell subpopulations.Plast Reconstr Surg. 2011 Sep;128(3):663-672. doi: 10.1097/PRS.0b013e318221db33. Plast Reconstr Surg. 2011. PMID: 21572381 Free PMC article.
-
Defining adipose tissue-derived stem cells in tissue and in culture.Histol Histopathol. 2010 Jun;25(6):807-15. doi: 10.14670/HH-25.807. Histol Histopathol. 2010. PMID: 20376787 Review.
-
Cryopreserved Adipose Tissue-Derived Stromal/Stem Cells: Potential for Applications in Clinic and Therapy.Adv Exp Med Biol. 2016;951:137-146. doi: 10.1007/978-3-319-45457-3_11. Adv Exp Med Biol. 2016. PMID: 27837560 Review.
Cited by
-
Mechanical and Enzymatic Digestion of Autologous Fat Grafting (A-FG): Fat Volume Maintenance and AD-SVFs Amount in Comparison.Aesthetic Plast Surg. 2024 Jan;48(1):27-28. doi: 10.1007/s00266-023-03463-3. Epub 2023 Jul 12. Aesthetic Plast Surg. 2024. PMID: 37438669 No abstract available.
-
Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting.J Clin Med. 2024 May 21;13(11):3028. doi: 10.3390/jcm13113028. J Clin Med. 2024. PMID: 38892739 Free PMC article.
-
Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking.Int J Mol Sci. 2024 Oct 16;25(20):11124. doi: 10.3390/ijms252011124. Int J Mol Sci. 2024. PMID: 39456905 Free PMC article. Review.
-
Evaluation of a Novel Mechanical Device for the Production of Microfragmented Adipose Tissue for Veterinary Regenerative Medicine: A Proof-of-Concept.Int J Mol Sci. 2024 Nov 4;25(21):11854. doi: 10.3390/ijms252111854. Int J Mol Sci. 2024. PMID: 39519405 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

