Semaphorin 4D induced inhibitory synaptogenesis decreases epileptiform activity and alters progression to Status Epilepticus in mice
- PMID: 37163910
- PMCID: PMC10247425
- DOI: 10.1016/j.eplepsyres.2023.107156
Semaphorin 4D induced inhibitory synaptogenesis decreases epileptiform activity and alters progression to Status Epilepticus in mice
Abstract
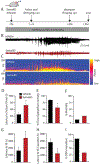
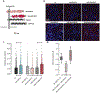
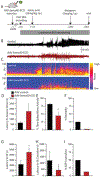
Previously we demonstrated that intra-hippocampal infusion of purified, Semaphorin 4D (Sema4D) extracellular domain (ECD) into the mouse hippocampus rapidly promotes formation of GABAergic synapses and decreases seizure susceptibility in mice. Given the relatively fast action of Sema4D treatment revealed by these studies, we sought to determine the time course of Sema4D treatment on hippocampal network activity using an acute hippocampal slice preparation. We performed long-term extracellular recordings from area CA1 encompassing a 2-hour application of Sema4D and found that hippocampal excitation is suppressed for hours following treatment. We also asked if Sema4D treatment could ameliorate seizures in an acute seizure model: the kainic acid (KA) mouse model. We demonstrate that Sema4D treatment delays and suppresses ictal activity, delays the transition to Status Epilepticus (SE), and lessens the severity of SE. Lastly, we sought to explore alternative methods of Sema4D delivery to hippocampus and thus created an Adeno Associated Virus expressing the ECD of Sema4D. Our data reveal that virally delivered, chronically overexpressed Sema4D-ECD promotes GABAergic synapse formation and suppresses ictal activity and progression to SE. These results provide proof of concept that viral delivery of Sema4D is an efficacious and promising delivery method to abate epileptiform activity and progression to SE.
Keywords: GABAergic synapse; Hippocampal inhibition; Refractory epilepsy; Sema4D; Status epilepticus.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Author Suzanne Paradis holds US Patent US-10626163-B2 entitled "Methods of Modulating GABAergic Inhibitory Synapse Formation and Function Using Sema4D." Co-inventors: Kuzirian, Marissa; Moore, Anna; Paradis, Suzanne. Author Suzanne Paradis is also co-founder and President of Severin Therapeutics, Inc. Author Jamie Maguire is on the Scientific Advisory Board for SAGE Therapeutics. The remaining authors have no conflicts of interest to disclose.
Figures
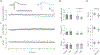
Similar articles
-
Semaphorin 4D promotes inhibitory synapse formation and suppresses seizures in vivo.Epilepsia. 2018 Jun;59(6):1257-1268. doi: 10.1111/epi.14429. Epub 2018 May 25. Epilepsia. 2018. PMID: 29799628 Free PMC article.
-
The class 4 semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus.J Neurosci. 2013 May 22;33(21):8961-73. doi: 10.1523/JNEUROSCI.0989-13.2013. J Neurosci. 2013. PMID: 23699507 Free PMC article.
-
Sema4D localizes to synapses and regulates GABAergic synapse development as a membrane-bound molecule in the mammalian hippocampus.Mol Cell Neurosci. 2013 Nov;57:23-32. doi: 10.1016/j.mcn.2013.08.004. Epub 2013 Sep 10. Mol Cell Neurosci. 2013. PMID: 24036351 Free PMC article.
-
Receptor Functions of Semaphorin 4D.Biochemistry (Mosc). 2019 Sep;84(9):1021-1027. doi: 10.1134/S0006297919090049. Biochemistry (Mosc). 2019. PMID: 31693461 Review.
-
Semaphorin 4D as a guidance molecule in the immune system.Int Rev Immunol. 2021;40(4):268-273. doi: 10.1080/08830185.2021.1905807. Epub 2021 Mar 31. Int Rev Immunol. 2021. PMID: 33787446 Review.
Cited by
-
Plexin-B1 and Plexin-B2 play non-redundant roles in GABAergic synapse formation.Mol Cell Neurosci. 2024 Mar;128:103920. doi: 10.1016/j.mcn.2024.103920. Epub 2024 Feb 6. Mol Cell Neurosci. 2024. PMID: 38331011 Free PMC article.
-
Semaphorin heterodimerization in cis regulates membrane targeting and neocortical wiring.Nat Commun. 2024 Aug 16;15(1):7059. doi: 10.1038/s41467-024-51009-1. Nat Commun. 2024. PMID: 39152101 Free PMC article.
References
-
- Anderson WW, Collingridge GL, 2007. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods 162, 346–356. - PubMed
-
- Campbell I, 2007. Chi-squared and Fisher-lrwin tests of two-by-two tables with small sample recommendations. Statistics in Medicine 26, 3661–3675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

