Decoding hereditary spastic paraplegia pathogenicity through transcriptomic profiling
- PMID: 37161652
- PMCID: PMC10236304
- DOI: 10.24272/j.issn.2095-8137.2022.281
Decoding hereditary spastic paraplegia pathogenicity through transcriptomic profiling
Abstract
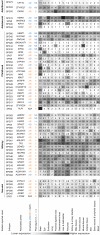
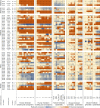
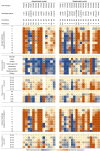
Hereditary spastic paraplegia (HSP) is a group of genetic motor neuron diseases resulting from length-dependent axonal degeneration of the corticospinal upper motor neurons. Due to the advancement of next-generation sequencing, more than 70 novel HSP disease-causing genes have been identified in the past decade. Despite this, our understanding of HSP physiopathology and the development of efficient management and treatment strategies remain poor. One major challenge in studying HSP pathogenicity is selective neuronal vulnerability, characterized by the manifestation of clinical symptoms that are restricted to specific neuronal populations, despite the presence of germline disease-causing variants in every cell of the patient. Furthermore, disease genes may exhibit ubiquitous expression patterns and involve a myriad of different pathways to cause motor neuron degeneration. In the current review, we explore the correlation between transcriptomic data and clinical manifestations, as well as the importance of interspecies models by comparing tissue-specific transcriptomic profiles of humans and mice, expression patterns of different genes in the brain during development, and single-cell transcriptomic data from related tissues. Furthermore, we discuss the potential of emerging single-cell RNA sequencing technologies to resolve unanswered questions related to HSP pathogenicity.
遗传性痉挛性截瘫(HSP)是一组由皮质脊髓上运动神经元的轴突变性引起的遗传性疾病。近年来,随着二代测序技术的发展,已发现70多个HSP致病基因。尽管如此,我们对HSP的致病机理及有效治疗手段尚无明确认知。 HSP的致病性研究面临着一个主要挑战,即病人表现出选择性神经元易感性;具体表现为,即使导致疾病的变异普遍存在于病人的每一个细胞内,其临床症状仅限于特定类型的神经元。此外,疾病基因可能在各种组织和细胞内均一表达,并涉及不同的信号通路,从而导致运动神经元的退行。该研究比较了人类和小鼠的组织特异性转录组学概况,以及发育过程中大脑内不同HSP基因的表达量,并使用单细胞RNA测序结果分析来自相关组织的细胞层面转录组数据。此外,我们还探讨了单细胞RNA测序技术在探究HSP致病机理方面的潜力。.
Keywords: Disease pathogenicity; Hereditary spastic paraplegia; Motor neuron degeneration; Single-cell RNA sequencing; Transcriptome analysis.
Figures

Similar articles
-
Genetic origin of patients having spastic paraplegia with or without other neurologic manifestations.BMC Neurol. 2022 May 16;22(1):180. doi: 10.1186/s12883-022-02708-z. BMC Neurol. 2022. PMID: 35578252 Free PMC article.
-
Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78).Brain. 2017 Feb;140(2):287-305. doi: 10.1093/brain/aww307. Brain. 2017. PMID: 28137957 Free PMC article.
-
Mutations in CAPN1 Cause Autosomal-Recessive Hereditary Spastic Paraplegia.Am J Hum Genet. 2016 May 5;98(5):1038-1046. doi: 10.1016/j.ajhg.2016.04.002. Am J Hum Genet. 2016. PMID: 27153400 Free PMC article.
-
Hereditary spastic paraplegia: More than an upper motor neuron disease.Rev Neurol (Paris). 2017 May;173(5):352-360. doi: 10.1016/j.neurol.2017.03.034. Epub 2017 Apr 24. Rev Neurol (Paris). 2017. PMID: 28449883 Review.
-
Ophthalmological changes in hereditary spastic paraplegia and other genetic diseases with spastic paraplegia.J Neurol Sci. 2020 Feb 15;409:116620. doi: 10.1016/j.jns.2019.116620. Epub 2019 Dec 6. J Neurol Sci. 2020. PMID: 31865189 Review.
Cited by
-
Jump further, leap higher, and consolidate stronger: A brief review of the long-term partnership between Kunming Institute of Zoology (KIZ) and the Chinese University of Hong Kong (CUHK) in bioresources and molecular research.Zool Res. 2023 May 18;44(3):556-558. doi: 10.24272/j.issn.2095-8137.2023.091. Zool Res. 2023. PMID: 37070588 Free PMC article. No abstract available.
References
-
-
Allen Institute. 2021. Cell types database: RNA-Seq data.https://portal.brain-map.org/atlases-and-data/rnaseq.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
