Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
- PMID: 37160880
- PMCID: PMC10169838
- DOI: 10.1038/s41467-023-37863-5
Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
Abstract
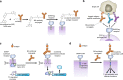
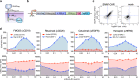
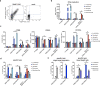
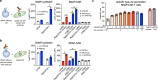
Chimeric antigen receptors (CARs) and synthetic Notch (synNotch) receptors are engineered cell-surface receptors that sense a target antigen and respond by activating T cell receptor signaling or a customized gene program, respectively. Here, to expand the targeting capabilities of these receptors, we develop "universal" receptor systems for which receptor specificity can be directed post-translationally via covalent attachment of a co-administered antibody bearing a benzylguanine (BG) motif. A SNAPtag self-labeling enzyme is genetically fused to the receptor and reacts with BG-conjugated antibodies for covalent assembly, programming antigen recognition. We demonstrate that activation of SNAP-CAR and SNAP-synNotch receptors can be successfully targeted by clinically relevant BG-conjugated antibodies, including anti-tumor activity of SNAP-CAR T cells in vivo in a human tumor xenograft mouse model. Finally, we develop a mathematical model to better define the parameters affecting universal receptor signaling. SNAP receptors provide a powerful strategy to post-translationally reprogram the targeting specificity of engineered cells.
© 2023. The Author(s).
Conflict of interest statement
J.L. and A.D. are inventors on a patent application filed by the University of Pittsburgh on the universal SNAP receptor technology described herein (WO2020072764A1). The remaining authors declare no competing interests.
Figures



Similar articles
-
Engineering Axl specific CAR and SynNotch receptor for cancer therapy.Sci Rep. 2018 Mar 1;8(1):3846. doi: 10.1038/s41598-018-22252-6. Sci Rep. 2018. PMID: 29497107 Free PMC article.
-
Development of CAR T Cells Expressing a Suicide Gene Plus a Chimeric Antigen Receptor Targeting Signaling Lymphocytic-Activation Molecule F7.Mol Ther. 2021 Feb 3;29(2):702-717. doi: 10.1016/j.ymthe.2020.10.008. Epub 2020 Oct 14. Mol Ther. 2021. PMID: 33129371 Free PMC article.
-
Combined CD28 and 4-1BB Costimulation Potentiates Affinity-tuned Chimeric Antigen Receptor-engineered T Cells.Clin Cancer Res. 2019 Jul 1;25(13):4014-4025. doi: 10.1158/1078-0432.CCR-18-2559. Epub 2019 Apr 12. Clin Cancer Res. 2019. PMID: 30979735 Free PMC article.
-
Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance.Front Immunol. 2018 Oct 12;9:2359. doi: 10.3389/fimmu.2018.02359. eCollection 2018. Front Immunol. 2018. PMID: 30369931 Free PMC article. Review.
-
Unlocking the potential of Tregs: innovations in CAR technology.Front Mol Biosci. 2023 Oct 12;10:1267762. doi: 10.3389/fmolb.2023.1267762. eCollection 2023. Front Mol Biosci. 2023. PMID: 37900916 Free PMC article. Review.
Cited by
-
Engineering signalling pathways in mammalian cells.Nat Biomed Eng. 2024 Dec;8(12):1523-1539. doi: 10.1038/s41551-024-01237-z. Epub 2024 Sep 5. Nat Biomed Eng. 2024. PMID: 39237709 Review.
-
Immunogenicity of CAR-T Cell Therapeutics: Evidence, Mechanism and Mitigation.Front Immunol. 2022 May 23;13:886546. doi: 10.3389/fimmu.2022.886546. eCollection 2022. Front Immunol. 2022. PMID: 35677038 Free PMC article. Review.
-
Electrophilic proximity-inducing synthetic adapters enhance universal T cell function by covalently enforcing immune receptor signaling.Mol Ther Oncol. 2024 Jun 24;32(3):200842. doi: 10.1016/j.omton.2024.200842. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39045028 Free PMC article.
-
Challenges and strategies in relation to effective CAR-T cell immunotherapy for solid tumors.Med Oncol. 2024 Apr 23;41(5):126. doi: 10.1007/s12032-024-02310-y. Med Oncol. 2024. PMID: 38652178 Review.
-
Modular Chimeric Antigen Receptor Systems for Universal CAR T Cell Retargeting.Int J Mol Sci. 2020 Sep 30;21(19):7222. doi: 10.3390/ijms21197222. Int J Mol Sci. 2020. PMID: 33007850 Free PMC article. Review.
References
-
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Disco. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

