Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis
- PMID: 37158107
- PMCID: PMC10330362
- DOI: 10.1161/CIRCULATIONAHA.122.061131
Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis
Abstract
Background: Shortly after birth, cardiomyocytes exit the cell cycle and cease proliferation. At present, the regulatory mechanisms for this loss of proliferative capacity are poorly understood. CBX7 (chromobox 7), a polycomb group (PcG) protein, regulates the cell cycle, but its role in cardiomyocyte proliferation is unknown.
Methods: We profiled CBX7 expression in the mouse hearts through quantitative real-time polymerase chain reaction, Western blotting, and immunohistochemistry. We overexpressed CBX7 in neonatal mouse cardiomyocytes through adenoviral transduction. We knocked down CBX7 by using constitutive and inducible conditional knockout mice (Tnnt2-Cre;Cbx7fl/+ and Myh6-MCM;Cbx7fl/fl, respectively). We measured cardiomyocyte proliferation by immunostaining of proliferation markers such as Ki67, phospho-histone 3, and cyclin B1. To examine the role of CBX7 in cardiac regeneration, we used neonatal cardiac apical resection and adult myocardial infarction models. We examined the mechanism of CBX7-mediated repression of cardiomyocyte proliferation through coimmunoprecipitation, mass spectrometry, and other molecular techniques.
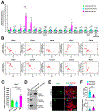
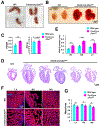
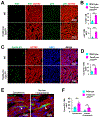
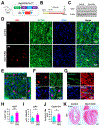
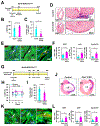
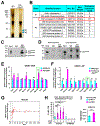
Results: We explored Cbx7 expression in the heart and found that mRNA expression abruptly increased after birth and was sustained throughout adulthood. Overexpression of CBX7 through adenoviral transduction reduced proliferation of neonatal cardiomyocytes and promoted their multinucleation. On the other hand, genetic inactivation of Cbx7 increased proliferation of cardiomyocytes and impeded cardiac maturation during postnatal heart growth. Genetic ablation of Cbx7 promoted regeneration of neonatal and adult injured hearts. Mechanistically, CBX7 interacted with TARDBP (TAR DNA-binding protein 43) and positively regulated its downstream target, RBM38 (RNA Binding Motif Protein 38), in a TARDBP-dependent manner. Overexpression of RBM38 inhibited the proliferation of CBX7-depleted neonatal cardiomyocytes.
Conclusions: Our results demonstrate that CBX7 directs the cell cycle exit of cardiomyocytes during the postnatal period by regulating its downstream targets TARDBP and RBM38. This is the first study to demonstrate the role of CBX7 in regulation of cardiomyocyte proliferation, and CBX7 could be an important target for cardiac regeneration.
Keywords: CBX7 protein; cell cycle; cell proliferation; guided tissue regeneration; human; myocytes, cardiac; polycomb-group proteins.
Conflict of interest statement
Figures
Comment in
-
Letter by Zhang et al Regarding Article, "Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis".Circulation. 2023 Nov 14;148(20):1602-1603. doi: 10.1161/CIRCULATIONAHA.123.066427. Epub 2023 Nov 13. Circulation. 2023. PMID: 37956225 No abstract available.
Similar articles
-
Response by Cho and Yoon to Letter Regarding Article, "Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis".Circulation. 2023 Nov 14;148(20):1604-1605. doi: 10.1161/CIRCULATIONAHA.123.066624. Epub 2023 Nov 13. Circulation. 2023. PMID: 37956224 No abstract available.
-
Letter by Zhang et al Regarding Article, "Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis".Circulation. 2023 Nov 14;148(20):1602-1603. doi: 10.1161/CIRCULATIONAHA.123.066427. Epub 2023 Nov 13. Circulation. 2023. PMID: 37956225 No abstract available.
-
RNA-Binding Protein LIN28a Regulates New Myocyte Formation in the Heart Through Long Noncoding RNA-H19.Circulation. 2023 Jan 24;147(4):324-337. doi: 10.1161/CIRCULATIONAHA.122.059346. Epub 2022 Oct 31. Circulation. 2023. PMID: 36314132 Free PMC article.
-
Promoting cardiomyocyte proliferation for myocardial regeneration in large mammals.J Mol Cell Cardiol. 2024 Mar;188:52-60. doi: 10.1016/j.yjmcc.2024.01.005. Epub 2024 Feb 9. J Mol Cell Cardiol. 2024. PMID: 38340541 Review.
-
A change of heart: understanding the mechanisms regulating cardiac proliferation and metabolism before and after birth.J Physiol. 2023 Apr;601(8):1319-1341. doi: 10.1113/JP284137. Epub 2023 Mar 13. J Physiol. 2023. PMID: 36872609 Free PMC article. Review.
Cited by
-
Macrophage-driven cardiac inflammation and healing: insights from homeostasis and myocardial infarction.Cell Mol Biol Lett. 2023 Oct 19;28(1):81. doi: 10.1186/s11658-023-00491-4. Cell Mol Biol Lett. 2023. PMID: 37858035 Free PMC article. Review.
-
Targeting cardiomyocyte cell cycle regulation in heart failure.Basic Res Cardiol. 2024 Jun;119(3):349-369. doi: 10.1007/s00395-024-01049-x. Epub 2024 Apr 29. Basic Res Cardiol. 2024. PMID: 38683371 Free PMC article. Review.
-
Glycation in the cardiomyocyte.Vitam Horm. 2024;125:47-88. doi: 10.1016/bs.vh.2024.04.005. Epub 2024 May 24. Vitam Horm. 2024. PMID: 38997172 Review.
References
-
- Braunwald E The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–24. - PubMed
-
- Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC and McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

