CD44 and HAP-Conjugated hADSCs as Living Materials for Targeted Tumor Therapy and Bone Regeneration
- PMID: 37156753
- PMCID: PMC10369264
- DOI: 10.1002/advs.202206393
CD44 and HAP-Conjugated hADSCs as Living Materials for Targeted Tumor Therapy and Bone Regeneration
Abstract
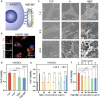
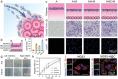
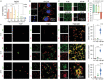
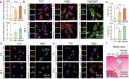
Combining targeted tumor therapy with tissue regeneration represents a promising strategy for synergistic tumor therapy. In this study, a multifunctional living material is constructed with human-derived adipose stem cells (hADSCs) and antibody-modified hydroxyapatite nanorods (nHAP) for targeted drug delivery and bone regeneration following surgery. The living material delivers the therapeutics to the tumor site efficiently based on the strength of the inherent tumor tropism of hADSCs. The bioconjugation of nHAP with hADSCs via specific antibody modification is found to be biocompatible, even when loaded with the chemotherapeutic drug doxorubicin (Dox). The endocytosis of nHAP stimulates the osteogenic differentiation of hADSCs, promoting bone tissue regeneration. Moreover, the antibody-modified nHAP-hADSC conjugate exhibits targeted tumor delivery, which is further facilitated by pH-triggered release of Dox, inducing apoptosis of tumor cells with low toxicity to healthy tissues. Therefore, the present study provides a general strategy for engineering living materials to achieve targeted tumor therapy and bone tissue regeneration after surgery, which can be extended to other disease types.
Keywords: bone tissue regeneration; living material; stem cells; targeted tumor therapy.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
One-Dimensional Hydroxyapatite Nanostructures with Tunable Length for Efficient Stem Cell Differentiation Regulation.ACS Appl Mater Interfaces. 2017 Oct 4;9(39):33717-33727. doi: 10.1021/acsami.7b13313. Epub 2017 Sep 22. ACS Appl Mater Interfaces. 2017. PMID: 28906099
-
The effect of epigenetic reprogramming using MI192 HDAC inhibitor on enhancing the osteogenesis of human adipose-derived stem cells in vitro.Biosci Rep. 2023 May 31;43(5):BSR20221635. doi: 10.1042/BSR20221635. Biosci Rep. 2023. PMID: 37022380 Free PMC article.
-
Nanostructured Tendon-Derived Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells.ACS Appl Mater Interfaces. 2016 Sep 7;8(35):22819-29. doi: 10.1021/acsami.6b05358. Epub 2016 Aug 23. ACS Appl Mater Interfaces. 2016. PMID: 27502160
-
Transcriptomic analysis and biological evaluation reveals that LMO3 regulates the osteogenic differentiation of human adipose derived stem cells via PI3K/Akt signaling pathway.J Mol Histol. 2022 Apr;53(2):379-394. doi: 10.1007/s10735-021-10047-5. Epub 2022 Feb 14. J Mol Histol. 2022. PMID: 35165791
-
Sericin/Nano-Hydroxyapatite Hydrogels Based on Graphene Oxide for Effective Bone Regeneration via Immunomodulation and Osteoinduction.Int J Nanomedicine. 2023 Apr 6;18:1875-1895. doi: 10.2147/IJN.S399487. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37051313 Free PMC article.
Cited by
-
Engineered nanoplatform mediated gas therapy enhanced ferroptosis for tumor therapy in vivo.Bioact Mater. 2024 Nov 5;44:488-500. doi: 10.1016/j.bioactmat.2024.10.024. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39559423 Free PMC article.
-
CD44 and its implication in neoplastic diseases.MedComm (2020). 2024 May 23;5(6):e554. doi: 10.1002/mco2.554. eCollection 2024 Jun. MedComm (2020). 2024. PMID: 38783892 Free PMC article. Review.
References
-
- Gill J., Gorlick R., Nat. Rev. Clin. Oncol. 2021, 18, 609. - PubMed
-
- Gianferante D. M., Mirabello L., Savage S. A., Nat. Rev. Endocrinol. 2017, 13, 480. - PubMed
-
- Long J., Zhang W., Chen Y., Teng B., Liu B., Li H., Yao Z., Wang D., Li L., Yu X. F., Qin L., Lai Y., Biomaterials 2021, 275, 120950. - PubMed
-
- Kansara M., Teng M. W., Smyth M. J., Thomas D. M., Nat. Rev. Cancer 2014, 14, 722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
