Reduced pain sensitivity of episodic pain syndrome model mice carrying a Nav1.9 mutation by ANP-230, a novel sodium channel blocker
- PMID: 37151704
- PMCID: PMC10161610
- DOI: 10.1016/j.heliyon.2023.e15423
Reduced pain sensitivity of episodic pain syndrome model mice carrying a Nav1.9 mutation by ANP-230, a novel sodium channel blocker
Abstract
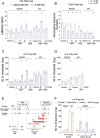
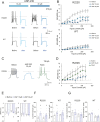
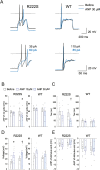
The sodium channel Nav1.9 is expressed in the sensory neurons of small diameter dorsal root ganglia that transmit pain signals, and gain-of-function Nav1.9 mutations have been associated with both painful and painless disorders. We initially determined that some Nav1.9 mutations are responsible for familial episodic pain syndrome observed in the Japanese population. We therefore generated model mice harboring one of the more painful Japanese mutations, R222S, and determined that dorsal root ganglia hyperexcitability was the cause of the associated pain. ANP-230 is a novel non-opioid drug with strong inhibitory effects on Nav1.7, 1.8 and 1.9, and is currently under clinical trials for patients suffering from familial episodic pain syndrome. However, little is known about its mechanism of action and effects on pain sensitivity. In this study, we therefore investigated the inhibitory effects of ANP-230 on the hypersensitivity of Nav1.9 p.R222S mutant model mouse to pain. In behavioral tests, ANP-230 reduced the pain response of the mice, particularly to heat or mechanical stimuli, in a concentration- and time-dependent manner. Furthermore, ANP-230 suppressed the repetitive firing of dorsal root ganglion neurons of these mutant mice. Our results clearly demonstrate that ANP-230 is an effective analgesic for familial episodic pain syndrome resulting from DRG neuron hyperexcitability, and that such analgesic effects are likely to be of clinical significance.
Keywords: Behavioral experiments; Electrophysiology; Episodic pain syndrome; Nav1.9 voltage-gated sodium channel; Non-opioid analgesics; Pain model mice; Pharmacology; Sodium channel blocker.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Infantile Pain Episodes Associated with Novel Nav1.9 Mutations in Familial Episodic Pain Syndrome in Japanese Families.PLoS One. 2016 May 25;11(5):e0154827. doi: 10.1371/journal.pone.0154827. eCollection 2016. PLoS One. 2016. PMID: 27224030 Free PMC article. Clinical Trial.
-
Mechanical allodynia triggered by cold exposure in mice with the Scn11a p.R222S mutation: a novel model of drug therapy for neuropathic pain related to NaV1.9.Naunyn Schmiedebergs Arch Pharmacol. 2021 Feb;394(2):299-306. doi: 10.1007/s00210-020-01978-z. Epub 2020 Sep 24. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 32970203 Free PMC article.
-
Increased Resurgent Sodium Currents in Nav1.8 Contribute to Nociceptive Sensory Neuron Hyperexcitability Associated with Peripheral Neuropathies.J Neurosci. 2019 Feb 20;39(8):1539-1550. doi: 10.1523/JNEUROSCI.0468-18.2018. Epub 2019 Jan 7. J Neurosci. 2019. PMID: 30617209 Free PMC article.
-
Painful and painless mutations of SCN9A and SCN11A voltage-gated sodium channels.Pflugers Arch. 2020 Jul;472(7):865-880. doi: 10.1007/s00424-020-02419-9. Epub 2020 Jun 29. Pflugers Arch. 2020. PMID: 32601768 Free PMC article. Review.
-
Painful and painless channelopathies.Lancet Neurol. 2014 Jun;13(6):587-99. doi: 10.1016/S1474-4422(14)70024-9. Epub 2014 May 6. Lancet Neurol. 2014. PMID: 24813307 Review.
References
LinkOut - more resources
Full Text Sources

