Does type of funding affect reporting in network meta-analysis? A scoping review of network meta-analyses
- PMID: 37149700
- PMCID: PMC10163730
- DOI: 10.1186/s13643-023-02235-z
Does type of funding affect reporting in network meta-analysis? A scoping review of network meta-analyses
Abstract
Background: Evidence has shown that private industry-sponsored randomized controlled trials (RCTs) and meta-analyses are more likely to report intervention-favourable results compared with other sources of funding. However, this has not been assessed in network meta-analyses (NMAs).
Objectives: To (a) explore the recommendation rate of industry-sponsored NMAs on their company's intervention, and (b) assess reporting in NMAs of pharmacologic interventions according to their funding type.
Methods: Design: Scoping review of published NMAs with RCTs.
Information sources: We used a pre-existing NMA database including 1,144 articles from MEDLINE, EMBASE and Cochrane Database of Systematic Reviews, published between January 2013 and July 2018.
Study selection: NMAs with transparent funding information and comparing pharmacologic interventions with/without placebo.
Synthesis: We captured whether NMAs recommended their own or another company's intervention, classified NMAs according to their primary outcome findings (i.e., statistical significance and direction of effect), and according to the overall reported conclusion. We assessed reporting using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension to NMA (PRISMA-NMA) 32-item checklist. We matched and compared industry with non-industry NMAs having the same research question, disease, primary outcome, and pharmacologic intervention against placebo/control.
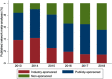
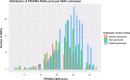
Results: We retrieved 658 NMAs, which reported a median of 23 items in the PRISMA-NMA checklist (interquartile range [IQR]: 21-26). NMAs were categorized as 314 publicly-sponsored (PRISMA-NMA median 24.5, IQR 22-27), 208 non-sponsored (PRISMA-NMA median 23, IQR 20-25), and 136 industry/mixed-sponsored NMAs (PRISMA-NMA median 21, IQR 19-24). Most industry-sponsored NMAs recommended their own manufactured drug (92%), suggested a statistically significant positive treatment-effect for their drug (82%), and reported an overall positive conclusion (92%). Our matched NMAs (25 industry vs 25 non-industry) indicated that industry-sponsored NMAs had favourable conclusions more often (100% vs 80%) and were associated with larger (but not statistically significantly different) efficacy effect sizes (in 61% of NMAs) compared with non-industry-sponsored NMAs.
Conclusions: Differences in completeness of reporting and author characteristics were apparent among NMAs with different types of funding. Publicly-sponsored NMAs had the best reporting and published their findings in higher impact-factor journals. Knowledge users should be mindful of this potential funding bias in NMAs.
Keywords: Funding bias; Industry-funding; Multiple treatment meta-analysis; Network meta-analysis; Sponsorship.
© 2023. The Author(s).
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Do reporting guidelines have an impact? Empirical assessment of changes in reporting before and after the PRISMA extension statement for network meta-analysis.Syst Rev. 2021 Sep 10;10(1):246. doi: 10.1186/s13643-021-01780-9. Syst Rev. 2021. PMID: 34507621 Free PMC article.
-
Reporting quality of systematic reviews with network meta-analyses in Endodontics.Clin Oral Investig. 2023 Jul;27(7):3437-3445. doi: 10.1007/s00784-023-04948-w. Epub 2023 Mar 13. Clin Oral Investig. 2023. PMID: 36914841
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Assessing the methodological and reporting quality of network meta-analyses in Chinese medicine.Medicine (Baltimore). 2018 Nov;97(47):e13052. doi: 10.1097/MD.0000000000013052. Medicine (Baltimore). 2018. PMID: 30461607 Free PMC article. Review.
-
Evaluation of the Reporting Standard Guidelines of Network Meta-Analyses in Physical Therapy: A Systematic Review.Healthcare (Basel). 2022 Nov 25;10(12):2371. doi: 10.3390/healthcare10122371. Healthcare (Basel). 2022. PMID: 36553895 Free PMC article. Review.
Cited by
-
Differences in the reporting of conflicts of interest and sponsorships in systematic reviews with meta-analyses in dentistry: an examination of factors associated with their reporting.Res Integr Peer Rev. 2024 Sep 30;9(1):10. doi: 10.1186/s41073-024-00150-y. Res Integr Peer Rev. 2024. PMID: 39350298 Free PMC article.
References
-
- Austin D, Hayford T. Research and Development in the Pharmaceutical Industry. Congressional Budget Office report. 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources