Reprogramming viral immune evasion for a rational design of next-generation vaccines for RNA viruses
- PMID: 37138878
- PMCID: PMC10149994
- DOI: 10.3389/fimmu.2023.1172000
Reprogramming viral immune evasion for a rational design of next-generation vaccines for RNA viruses
Abstract
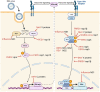
Type I interferons (IFNs-α/β) are antiviral cytokines that constitute the innate immunity of hosts to fight against viral infections. Recent studies, however, have revealed the pleiotropic functions of IFNs, in addition to their antiviral activities, for the priming of activation and maturation of adaptive immunity. In turn, many viruses have developed various strategies to counteract the IFN response and to evade the host immune system for their benefits. The inefficient innate immunity and delayed adaptive response fail to clear of invading viruses and negatively affect the efficacy of vaccines. A better understanding of evasion strategies will provide opportunities to revert the viral IFN antagonism. Furthermore, IFN antagonism-deficient viruses can be generated by reverse genetics technology. Such viruses can potentially serve as next-generation vaccines that can induce effective and broad-spectrum responses for both innate and adaptive immunities for various pathogens. This review describes the recent advances in developing IFN antagonism-deficient viruses, their immune evasion and attenuated phenotypes in natural host animal species, and future potential as veterinary vaccines.
Keywords: IFN antagonism; NF-kappa B (NF-κB); live-attenuated vaccine; next-generation vaccines; type I interferons (IFNs); veterinary vaccine; veterinary virology; viral immune evasion.
Copyright © 2023 Su, Du, Rowland, Wang and Yoo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
SARS-CoV-2 Evasion of the Interferon System: Can We Restore Its Effectiveness?Int J Mol Sci. 2023 May 27;24(11):9353. doi: 10.3390/ijms24119353. Int J Mol Sci. 2023. PMID: 37298304 Free PMC article. Review.
-
Interferon induction by RNA viruses and antagonism by viral pathogens.Viruses. 2014 Dec 12;6(12):4999-5027. doi: 10.3390/v6124999. Viruses. 2014. PMID: 25514371 Free PMC article. Review.
-
Human Cytomegalovirus DNA Polymerase Subunit UL44 Antagonizes Antiviral Immune Responses by Suppressing IRF3- and NF-κB-Mediated Transcription.J Virol. 2019 May 15;93(11):e00181-19. doi: 10.1128/JVI.00181-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30867312 Free PMC article.
-
Molecular Mechanisms of Foot-and-Mouth Disease Virus Targeting the Host Antiviral Response.Front Cell Infect Microbiol. 2017 Jun 13;7:252. doi: 10.3389/fcimb.2017.00252. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28660175 Free PMC article. Review.
-
Anti-chicken type I IFN countermeasures by major avian RNA viruses.Virus Res. 2020 Sep;286:198061. doi: 10.1016/j.virusres.2020.198061. Epub 2020 Jun 16. Virus Res. 2020. PMID: 32561378 Review.
Cited by
-
Suppression of TRIM19 by arterivirus nonstructural protein 1 promotes viral replication.Virus Res. 2024 Feb;340:199302. doi: 10.1016/j.virusres.2023.199302. Epub 2023 Dec 21. Virus Res. 2024. PMID: 38104946 Free PMC article.
-
Role of interferons in the antiviral battle: from virus-host crosstalk to prophylactic and therapeutic potential in SARS-CoV-2 infection.Front Immunol. 2024 Jan 15;14:1273604. doi: 10.3389/fimmu.2023.1273604. eCollection 2023. Front Immunol. 2024. PMID: 38288121 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

