Effects of neonatal nutrition interventions on neonatal mortality and child health and development outcomes: A systematic review
- PMID: 37133295
- PMCID: PMC8356300
- DOI: 10.1002/cl2.1141
Effects of neonatal nutrition interventions on neonatal mortality and child health and development outcomes: A systematic review
Abstract
Background: The last two decades have seen a significant decrease in mortality for children <5 years of age in low and middle-income countries (LMICs); however, neonatal (age, 0-28 days) mortality has not decreased at the same rate. We assessed three neonatal nutritional interventions that have the potential of reducing morbidity and mortality during infancy in LMICs.
Objectives: To determine the efficacy and effectiveness of synthetic vitamin A, dextrose oral gel, and probiotic supplementation during the neonatal period.
Search methods: We conducted electronic searches for relevant studies on the following databases: PubMed, CINAHL, LILACS, SCOPUS, and CENTRAL, Cochrane Central Register for Controlled Trials, up to November 27, 2019.
Selection criteria: We aimed to include randomized and quasi-experimental studies. The target population was neonates in LMICs. The interventions included synthetic vitamin A supplementation, oral dextrose gel supplementation, and probiotic supplementation during the neonatal period. We included studies from the community and hospital settings irrespective of the gestational age or birth weight of the neonate.
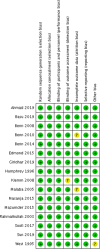
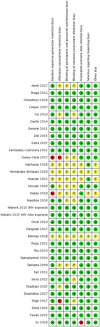
Data collection and analysis: Two authors screened the titles and extracted the data from selected studies. The risk of bias (ROB) in the included studies was assessed according to the Cochrane Handbook of Systematic Reviews. The primary outcome was all-cause mortality. The secondary outcomes were neonatal sepsis, necrotizing enterocolitis (NEC), prevention and treatment of neonatal hypoglycaemia, adverse events, and neurodevelopmental outcomes. Data were meta-analyzed by random effect models to obtain relative risk (RR) and 95% confidence interval (CI) for dichotomous outcomes and mean difference with 95% CI for continuous outcomes. The overall rating of evidence was determined by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Main results: Sixteen randomized studies (total participants 169,366) assessed the effect of vitamin A supplementation during the neonatal period. All studies were conducted in low- and middle-income (LMIC) countries. Thirteen studies were conducted in the community setting and three studies were conducted in the hospital setting, specifically in neonatal intensive care units. Studies were conducted in 10 different countries including India (four studies), Guinea-Bissau (three studies), Bangladesh (two studies), and one study each in China, Ghana, Indonesia, Nepal, Pakistan, Tanzania, and Zimbabwe. The overall ROB was low in most of the included studies for neonatal vitamin A supplementation. The pooled results from the community based randomized studies showed that there was no significant difference in all-cause mortality in the vitamin A (intervention) group compared to controls at 1 month (RR, 0.99; 95% CI, 0.90-1.08; six studies with 126,548 participants, statistical heterogeneity I 2 0%, funnel plot symmetrical, grade rating high), 6 months (RR, 0.98; 95% CI, 0.89-1.07; 12 studies with 154,940 participants, statistical heterogeneity I 2 43%, funnel plot symmetrical, GRADE quality high) and 12 months of age (RR, 1.04; 95% CI, 0.94-1.14; eight studies with 118,376 participants, statistical heterogeneity I 2 46%, funnel plot symmetrical, GRADE quality high). Neonatal vitamin A supplementation increased the incidence of bulging fontanelle by 53% compared to control (RR, 1.53; 95% CI, 1.12-2.09; six studies with 100,256 participants, statistical heterogeneity I 2 65%, funnel plot symmetrical, GRADE quality high). We did not identify any experimental study that addressed the use of dextrose gel for the prevention and/or treatment of neonatal hypoglycaemia in LMIC. Thirty-three studies assessed the effect of probiotic supplementation during the neonatal period (total participants 11,595; probiotics: 5854 and controls: 5741). All of the included studies were conducted in LMIC and were randomized. Most of the studies were done in the hospital setting and included participants who were preterm (born < 37 weeks gestation) and/or low birth weight (<2500 g birth weight). Studies were conducted in 13 different countries with 10 studies conducted in India, six studies in Turkey, three studies each in China and Iran, two each in Mexico and South Africa, and one each in Bangladesh, Brazil, Colombia, Indonesia, Nepal, Pakistan, and Thailand. Three studies were at high ROB due to lack of appropriate randomization sequence or allocation concealment. Combined data from 25 studies showed that probiotic supplementation reduced all-cause mortality by 20% compared to controls (RR, 0.80; 95% CI, 0.66-0.96; total number of participants 10,998, number needed to treat 100, statistical heterogeneity I 2 0%, funnel plot symmetrical, GRADE quality high). Twenty-nine studies reported the effect of probiotics on the incidence of NEC, and the combined results showed a relative reduction of 54% in the intervention group compared to controls (RR, 0.46; 95% CI, 0.35-0.59; total number of participants 5574, number needed to treat 17, statistical heterogeneity I 2 24%, funnel plot symmetrical, GRADE quality high). Twenty-one studies assessed the effect of probiotic supplementation during the neonatal period on neonatal sepsis, and the combined results showed a relative reduction of 22% in the intervention group compared to controls (RR, 0.78; 95% CI, 0.70-0.86; total number of participants 9105, number needed to treat 14, statistical heterogeneity I 2 23%, funnel plot symmetrical, GRADE quality high).
Authors' conclusions: Vitamin A supplementation during the neonatal period does not reduce all-cause neonatal or infant mortality in LMICs in the community setting. However, neonatal vitamin A supplementation increases the risk of Bulging Fontanelle. No experimental or quasi-experimental studies were available from LMICs to assess the effect of dextrose gel supplementation for the prevention or treatment of neonatal hypoglycaemia. Probiotic supplementation during the neonatal period seems to reduce all-cause mortality, NEC, and sepsis in babies born with low birth weight and/or preterm in the hospital setting. There was clinical heterogeneity in the use of probiotics, and we could not recommend any single strain of probiotics for wider use based on these results. There was a lack of studies on probiotic supplementation in the community setting. More research is needed to assess the effect of probiotics administered to neonates in-home/community setting in LMICs.
© 2021 The Authors. Campbell Systematic Reviews published by John Wiley & Sons Ltd on behalf of The Campbell Collaboration.
Figures


Update of
- Protocol
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries.Nutrients. 2020 Mar 17;12(3):791. doi: 10.3390/nu12030791. Nutrients. 2020. PMID: 32192165 Free PMC article.
-
Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2018 Dec 12;12(12):CD012519. doi: 10.1002/14651858.CD012519.pub2. Cochrane Database Syst Rev. 2018. PMID: 30548483 Free PMC article.
-
Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low- and middle-income countries: A systematic review.Campbell Syst Rev. 2021 May 5;17(2):e1156. doi: 10.1002/cl2.1156. eCollection 2021 Jun. Campbell Syst Rev. 2021. PMID: 37131925 Free PMC article. Review.
-
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2017 Jun 28;6(6):CD007137. doi: 10.1002/14651858.CD007137.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Mar 31;3:CD007137. doi: 10.1002/14651858.CD007137.pub6. PMID: 28658720 Free PMC article. Updated. Review.
References
REFERENCES TO STUDIES
INCLUDED STUDIES
-
- Aage, S. , Kiraly, N. , Da Costa, K. , Byberg, S. , BjerregaardAndersen, M. , Fisker, A. B. , Aaby, P. , & Benn, C. S. (2015). Neonatal vitamin A supplementation associated with increased atopy in girls. Allergy, 70(8), 985–994. - PubMed
-
- Agoestina, T. , Humphrey, J. H. , Taylor, G. A. , Usman, A. , Subardja, D. , Hidayat, S. , Nurachim, M. , Wu, L. , Friedman, D. S. , & West, K. P., Jr (1994). Safety of one 52‐mumol (50,000 IU) oral dose of vitamin A administered to neonates. Bulletin of the World Health Organization, 72(6), 859–868. - PMC - PubMed
-
- Ahmad, S. M. , Raqib, R. , Huda, M. N. , Alam, M. J. , Monirujjaman, M. , Akhter, T. , Wagatsuma, Y. , Qadri, F. , Zerofsky, M. S. , & Stephensen, C. B. (2019). High‐dose neonatal vitamin A supplementation transiently decreases thymic function in early infancy. Journal of Nutrition, 150(1), 176–183. - PMC - PubMed
-
- Ahmad, S. M. , Raqib, R. , Qadri, F. , & Stephensen, C. B. (2014). The effect of newborn vitamin A supplementation on infant immune functions: trial design, interventions, and baseline data. Contemporary Clinical Trials, 39(2), 269–279. - PubMed
-
- Ali, H. , Hamadani, J. , Mehra, S. , Tofail, F. , Hasan, M. I. , Shaikh, S. , Shamim, A. A. , Wu, L. S. , West, K. P., Jr , & Christian, P. (2017). Effect of maternal antenatal and newborn supplementation with vitamin A on cognitive development of school‐aged children in rural Bangladesh: A follow‐up of a placebo‐controlled, randomized trial. American Journal of Clinical Nutrition, 106(1), 77–87. - PubMed
PUBLISHED AND UNPUBLISHED DATA
-
- Akar, M. , Eras, Z. , Oncel, M. Y. , Arayici, S. , Guzoglu, N. , Canpolat, F. E. , Uras, N. , & Oguz, S. S. (2017). Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. The Journal of Maternal‐Fetal & Neonatal Medicine, 30(4), 411–415. - PubMed
-
- Bahl, R. , Bhandari, N. , Dube, B. , Edmond, K. , Fawzi, W. , Fontaine, O. , Kaur, J. , Kirkwood, B. R. , Martines, J. , Masanja, H. , Mazumder, S. , Msham, S. , Newton, S. , Oleary, M. , Ruben, J. , Shannon, C. , Smith, E. , Taneja, S. , & Yoshida, S. (2012). Efficacy of early neonatal vitamin A supplementation in reducing mortality during infancy in Ghana, India and Tanzania: Study protocol for a randomized controlled trial. Trials, 13(22), 22. - PMC - PubMed
-
- Bahl, R. , Bhandari, N. , Dube, B. , Edmond, K. , Fawzi, W. , Fontaine, O. , Kaur, J. , Kirkwood, B. R. , Martines, J. , Masanja, H. , Mazumder, S. , Msham, S. , Newton, S. , Oleary, M. , Ruben, J. , Shannon, C. , Smith, E. , Taneja, S. , & Yoshida, S. (2012). Efficacy of early neonatal vitamin A supplementation in reducing mortality during infancy in Ghana, India and Tanzania: Study protocol for a randomized controlled trial. Trials, 13(22), 22. - PMC - PubMed
-
- Coles, C. L. , Rahmathullah, L. , Kanungo, R. , Thulasiraj, R. D. , Katz, J. , Santhosham, M. , & Tielsch, J. M. (2001). Vitamin A supplementation at birth delays pneumococcal colonization in South Indian infants. Journal of Nutrition, 131(2), 255–261. - PubMed
-
- Dongol Singh, S. S. , Klobassa, D. S. , Resch, B. , Urlesberger, B. , & Shrestha, R. P. (2017). Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at Dhulikhel Hospital in Nepal. Kathmandu University Medical Journal, 15(60), 319–323. - PubMed
EXCLUDED STUDIES
-
- Abdulkadir, B. , Nelson, A. , Skeath, T. , Marrs, E. C. , Perry, J. D. , Cummings, S. P. , Embleton, N. D. , Berrington, J. E. , & Stewart, C. J. (2016). Routine use of probiotics in preterm infants: Longitudinal impact on the microbiome and metabolome. Neonatology, 109(4), 293–247. - PubMed
-
- Abrahamse‐Berkeveld, M. , Alles, M. , Franke‐Beckmann, E. , Helm, K. , Knecht, R. , Köllges, R. , Sandner, B. , Knol, J. , Ben Amor, K. , & Bufe, A. (2016). Infant formula containing galacto‐and fructo‐oligosaccharides and Bifidobacterium breve M‐16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double‐blind, prospective, multicentre study. Journal of Nutritional Science, 28(5), e42. - PMC - PubMed
-
- Abrahamsson, T. , Jakobsson, T. , Sinkiewicz, G. , Fredriksson, M. , & Bjorksten, B. (2005). Intestinal microbiota in infants supplemented with the probiotic bacterium Lactobacillus reuteri . Journal of Pediatric Gastroenterology and Nutrition, 40(5), 692.
-
- ADAPTS trial. (2019). A randomised controlled trial: Effect of probiotics on gut microbiome and vaccine responses in newborns with antibiotic‐induced dysbiosis (ADAPTS: Antibiotic Dysbiosis and Probiotics Trial in infantS). ACTRN12619000369123p.
-
- Agarwal, R. , Sharma, N. , Chaudhry, R. , Deorari, A. , Paul, V. K. , Gewolb, I. H. , & Panigrahi, P. (2003). Effects of oral Lactobacillus GG on enteric microflora in low‐birth‐weight neonates. Journal of Pediatrics Gastroenterology and Nutrition, 36(3), 397–402. - PubMed
STUDIES AWAITING CLASSIFICATION
-
- Barclay, D. , Puccio, G. , Fazzolari‐Nesci, A. , Giammanco, A. , Raiha, N. , Carrie Fassler, A. L. , Brown, C. , Chauffard, F. , Grathwohl, D. , Hager, C. , & Endres, W. (2003). Growth and tolerance of a whey‐based starter infant formula with enhanced protein efficiency and containing pro‐, pre‐ or synbiotics. A randomized controlled trial in term infants. Journal of Pediatrics Gastroenterology and Nutrition, 37, 388.
-
- Chubarova, A. I. , & Sharyafetdinova, G. R. (2017). An experience of using a preparation containing combined probiotic strains of bifidobacteria and lactobacilli in premature newborns in neonatal resuscitation and intensive care units. Voprosy Detskoi Dietologii, 15(4), 5–13.
ONGOING STUDIES
-
- Del Piano, M. , Coggiola, F. , Pane, M. , Amoruso, A. , Nicola, S. , & Mogna, L. (2015). Can probiotics reduce diarrhea and infant mortality in africa?: The project of a pilot study. Journal of Clinical Gastroenterology, S120–S123. - PubMed
-
- Goodman, T. (2015). Prevention of vitamin A deficiency by supplementation alongside routine vaccinations: A randomised controlled trial in Ghana infants. http://isrctn.com/ISRCTN97670178ISRCTN97670178
-
- Kaur, A. (2018). Effect of probiotic supplementation on feed tolerance and weight gain in low birth weight infants on tube feeds. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26719
-
- Londhe, A. (2019). Use of zinc and pre‐probiotics as a therapeutic adjunct in neonatal sepsis in preterms—An Open label randomized controlled trial. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16167
-
- Mirmohammdi, M. F. (2018). The effect of probiotics on prevention of necrotizing enterocolitis in neonates. http://en.irct.ir/trial/25078IRCT20170121032075N2
OTHER REFERENCES—ADDITIONAL REFERENCES
-
- van den Akker, C. H. P. , van Goudoever, J. B. , Szajewska, H. , Embleton, N. D. , Hojsak, I. , Reid, D. , & Shamir, R. (2018). Probiotics for Preterm Infants: A strain specific systematic review and network meta‐analysis. Journal of Pediatric Gastroenterology and Nutrition, 67, 1–122. 10.1097/MPG.0000000000001897 - DOI - PubMed
-
- Alfaleh, K. , Anabrees, J. , Bassler, D. , & Al‐Kharfi, T. (2011). Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews, 16(3), CD005496. - PubMed
-
- Alkema, L. , Chao, F. , You, D. , Pedersen, J. , & Sawyer, C. (2014). National, regional, and global sex ratios of infant, child and under‐5 mortality and identification of countries with outlying ratios: a systematic assessment. The Lancet Global Health, 2(9), e521–e530. 10.1016/S2214-109X(14)70280-3 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
