Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-calcoaceticus Complex Infections
- PMID: 37125467
- PMCID: PMC10150276
- DOI: 10.1093/cid/ciad094
Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-calcoaceticus Complex Infections
Abstract
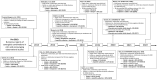
Carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex (CRAB) is one of the top-priority pathogens for new antibiotic development. Unlike other antibiotic-resistant threats, none of the available therapies have been shown to consistently reduce mortality or improve patient outcomes in clinical trials. Antibiotic combination therapy is routinely used in clinical practice; however, the preferred combination has not been defined. This narrative review focuses on evidence-based solutions for the treatment of invasive CRAB infections. We dissect the promise and perils of traditional agents used in combination, such as colistin, sulbactam, and the tetracyclines, and offer clinical pearls based on our interpretation of the available data. Next, we investigate the merits of newly developed β-lactam agents like cefiderocol and sulbactam-durlobactam, which have demonstrated contrasting results in recent randomized clinical trials. The review concludes with the authors' perspective on the evolving treatment landscape for CRAB infections, which is complicated by limited clinical data, imperfect treatment options, and a need for future clinical trials. We propose that effective treatment for CRAB infections requires a personalized approach that incorporates host factors, the site of infection, pharmacokinetic-pharmacodynamic principles, local molecular epidemiology of CRAB isolates, and careful interpretation of antibiotic susceptibility testing results. In most clinical scenarios, a dose-optimized, sulbactam-based regimen is recommended with the addition of at least one other in vitro active agent. Should sulbactam-durlobactam receive regulatory approval, recommendations will need to be re-evaluated with the most recent evidence.
Keywords: Acinetobacter; cefiderocol; colistin; durlobactam; sulbactam.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . R. K. S. has served as a consultant for Allergan, Cidara, Entasis, GlaxoSmithKline, Melinta, Menarini, Merck, Pifzer, Shionogi, Utility, and Venatorx, and has received investigator-initiated funding from Merck, Melinta, Roche, Shionogi, and Venatorx. D. L. P. has served as a consultant for Accelerate, Biomerieux, Entasis, Lysovant, Merck, Pfizer, Shionogi, and Venatorx, and has received research grants from Merck, Pfizer, and Shionogi. P. D. T. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Comment in
-
Intravenous Fosfomycin: The Underdog Player in the Treatment of Carbapenem-resistant Acinetobacter baumannii Infections.Clin Infect Dis. 2023 Dec 15;77(12):1736-1737. doi: 10.1093/cid/ciad435. Clin Infect Dis. 2023. PMID: 37477512 No abstract available.
Similar articles
-
In Vitro Activity of Sulbactam-Durlobactam against Global Isolates of Acinetobacter baumannii-calcoaceticus Complex Collected from 2016 to 2021.Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0078122. doi: 10.1128/aac.00781-22. Epub 2022 Aug 25. Antimicrob Agents Chemother. 2022. PMID: 36005804 Free PMC article.
-
Activity of Sulbactam-Durlobactam and Comparators Against a National Collection of Carbapenem-Resistant Acinetobacter baumannii Isolates From Greece.Front Cell Infect Microbiol. 2022 Jan 20;11:814530. doi: 10.3389/fcimb.2021.814530. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35127562 Free PMC article.
-
Successful Treatment of Carbapenem-Resistant Acinetobacter baumannii Meningitis With Sulbactam-Durlobactam.Clin Infect Dis. 2024 Oct 15;79(4):819-825. doi: 10.1093/cid/ciae210. Clin Infect Dis. 2024. PMID: 38630890
-
Sulbactam-Durlobactam in the Treatment of Carbapenem-Resistant Acinetobacter baumannii Infections.Ann Pharmacother. 2024 Jul;58(7):735-741. doi: 10.1177/10600280231204566. Epub 2023 Oct 10. Ann Pharmacother. 2024. PMID: 37817550 Review.
-
Sulbactam-durlobactam: A novel β-lactam-β-lactamase inhibitor combination targeting carbapenem-resistant Acinetobacter baumannii infections.Pharmacotherapy. 2023 Jun;43(6):502-513. doi: 10.1002/phar.2802. Epub 2023 May 23. Pharmacotherapy. 2023. PMID: 37052117 Review.
Cited by
-
New Agents Are Coming, and So Is the Resistance.Antibiotics (Basel). 2024 Jul 13;13(7):648. doi: 10.3390/antibiotics13070648. Antibiotics (Basel). 2024. PMID: 39061330 Free PMC article. Review.
-
Challenges Facing Two Outbreaks of Carbapenem-Resistant Acinetobacter baumannii: From Cefiderocol Susceptibility Testing to the Emergence of Cefiderocol-Resistant Mutants.Antibiotics (Basel). 2024 Aug 21;13(8):784. doi: 10.3390/antibiotics13080784. Antibiotics (Basel). 2024. PMID: 39200084 Free PMC article.
-
Optimizing Treatment for Carbapenem-Resistant Acinetobacter baumannii Complex Infections: A Review of Current Evidence.Infect Chemother. 2024 Jun;56(2):171-187. doi: 10.3947/ic.2024.0055. Infect Chemother. 2024. PMID: 38960737 Free PMC article. Review.
-
The Prevalence of Multidrug-Resistant Acinetobacter baumannii and Its Vaccination Status among Healthcare Providers.Vaccines (Basel). 2023 Jun 28;11(7):1171. doi: 10.3390/vaccines11071171. Vaccines (Basel). 2023. PMID: 37514987 Free PMC article. Review.
-
Comparison of cefiderocol and colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii: a systematic review with meta-analysis and trial sequential analysis.BMC Infect Dis. 2024 Sep 13;24(1):967. doi: 10.1186/s12879-024-09899-5. BMC Infect Dis. 2024. PMID: 39271977 Free PMC article.
References
-
- Tacconelli E, Carrara E, Savoldi A, et al. . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18(3):318–27. - PubMed
-
- . Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States . Available at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re.... Accessed 11 January 2022.
-
- Bassetti M, Echols R, Matsunaga Y, et al. . Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21(2):226–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

