Case report: Complete response of an anaplastic thyroid carcinoma patient with NRAS Q61R/ BRAF D594N mutations to the triplet of dabrafenib, trametinib and PD-1 antibody
- PMID: 37122752
- PMCID: PMC10140402
- DOI: 10.3389/fimmu.2023.1178682
Case report: Complete response of an anaplastic thyroid carcinoma patient with NRAS Q61R/ BRAF D594N mutations to the triplet of dabrafenib, trametinib and PD-1 antibody
Abstract
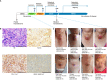
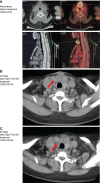
Anaplastic thyroid carcinoma, BRAF non-V600, NRAS, combination immunotherapy and targeted therapy, case report. Anaplastic thyroid carcinoma (ATC) is a rare type of thyroid cancer with a mortality rate near 100%. BRAF V600 and NRAS mutations are the most common drivers of ATC. While patients with BRAF V600-mutated ATC can be treated with BRAF-targeted therapy, there is no effective treatment for ATC driven by NRAS or non-V600 BRAF mutations. For patients with untargetable driver mutations, immunotherapy provides an alternative treatment option. Here, we present a metastatic ATC patient with PD-L1 positive (tumor proportion score of 60%) tumor and NRAS Q61R/BRAF D594N mutations, who progressed on PD-1 antibody sintilimab plus angiogenesis inhibitor anlotinib. The class 3 BRAF mutant D594N is sensitive to the inhibition of MEK inhibitor trametinib, and its oncogenic activity also depends on CRAF, which can be inhibited by BRAF inhibitor dabrafenib. For these reasons, the patient received a salvage treatment regime of dabrafenib, trametinib, and sintilimab, which resulted in a complete pathological response. To our best knowledge, this is the first report of successful treatment of ATC patients with concurrent NRAS/BRAF non-V600 mutations with the combination of immunotherapy and targeted therapy. Further investigation is required to decipher the mechanism by which the combination of dabrafenib/trametinib with PD-1 antibody overcomes initial immunotherapy resistance likely mediated by concurrent BRAF and NRAS mutations.
Keywords: BRAF non-V600E; NRAS; anaplastic thyroid carcinoma (ATC); case report; combination immunotherapy and targeted therapy.
Copyright © 2023 Gui, Zhu, Li, He, Ma, Cai and Liu.
Conflict of interest statement
XL and TM are employees of Genetron Health Beijing Technology, Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
Similar articles
-
Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study.Ann Oncol. 2022 Apr;33(4):406-415. doi: 10.1016/j.annonc.2021.12.014. Epub 2022 Jan 10. Ann Oncol. 2022. PMID: 35026411 Free PMC article. Clinical Trial.
-
Checkpoint Inhibition in Addition to Dabrafenib/Trametinib for BRAFV600E-Mutated Anaplastic Thyroid Carcinoma.Thyroid. 2024 Mar;34(3):336-346. doi: 10.1089/thy.2023.0573. Epub 2024 Feb 13. Thyroid. 2024. PMID: 38226606
-
Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells.Int J Oncol. 2016 Dec;49(6):2303-2308. doi: 10.3892/ijo.2016.3723. Epub 2016 Oct 7. Int J Oncol. 2016. PMID: 27748799
-
Rechallenge with dabrafenib plus trametinib in anaplastic thyroid cancer: A case report and review of literature.Curr Probl Cancer. 2021 Apr;45(2):100668. doi: 10.1016/j.currproblcancer.2020.100668. Epub 2020 Oct 22. Curr Probl Cancer. 2021. PMID: 33127167 Review.
-
Advances in the management of anaplastic thyroid carcinoma: transforming a life-threatening condition into a potentially treatable disease.Rev Endocr Metab Disord. 2024 Feb;25(1):123-147. doi: 10.1007/s11154-023-09833-1. Epub 2023 Aug 31. Rev Endocr Metab Disord. 2024. PMID: 37648897 Review.
Cited by
-
The role of targeted therapy and/or immunotherapy therapy in anaplastic thyroid carcinoma.Endocrine. 2024 Jun;84(3):1013-1020. doi: 10.1007/s12020-023-03647-6. Epub 2023 Dec 26. Endocrine. 2024. PMID: 38146047
-
Harnessing Immunity to Treat Advanced Thyroid Cancer.Vaccines (Basel). 2023 Dec 30;12(1):45. doi: 10.3390/vaccines12010045. Vaccines (Basel). 2023. PMID: 38250858 Free PMC article. Review.
References
-
- Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ book. Am Soc Clin Oncol Annu Meeting (2019) 39:147–64. doi: 10.1200/EDBK_240837 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

