In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform
- PMID: 37111339
- PMCID: PMC10141236
- DOI: 10.3390/ph16040582
In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform
Abstract
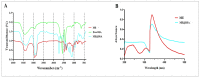
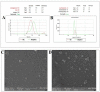
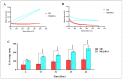
Combination of bovine serum albumin with microemulsions as constituting ingredient biopolymer has long been regarded an innovative method to address the surface functionalization and stability issues in the targeted payload deliveries, thereupon producing effectively modified microemulsions, which are superior in loading capacity, transitional and shelf-stability, as well as site-directed/site-preferred delivery, has become a favored option. The current study aimed to develop an efficient, suitable and functional microemulsion system encapsulating sesame oil (SO) as a model payload towards developing an efficient delivery platform. UV-VIS, FT-IR, and FE-SEM were used to characterize, and analyze the developed carrier. Physicochemical properties assessments of the microemulsion by dynamic light scattering size distributions, zeta-potential, and electron micrographic analyses were performed. The mechanical properties for rheological behavior were also studied. The HFF-2 cell line and hemolysis assays were conducted to ascertain the cell viability, and in vitro biocompatibility. The in vivo toxicity was determined based on a predicted median lethal dose (LD50) model, wherein the liver enzymes' functions were also tested to assess and confirm the predicted toxicity.
Keywords: LD50; biocompatibility; biological marker; bovine serum albumin; cytotoxicity; drug delivery; functional viability; hepatic functions; liver biomarkers; microemulsion; nanomedicine; nanoparticles; sesame oil; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In vivo and in vitro biocompatibility study of novel microemulsion hybridized with bovine serum albumin as nanocarrier for drug delivery.Heliyon. 2019 Jun 6;5(6):e01858. doi: 10.1016/j.heliyon.2019.e01858. eCollection 2019 Jun. Heliyon. 2019. PMID: 31198875 Free PMC article.
-
Microemulsion and bovine serum albumin nanoparticles as a novel hybrid nanocarrier system for efficient multifunctional drug delivery.J Biomed Mater Res A. 2020 Aug 1;108(8):1688-1702. doi: 10.1002/jbm.a.36935. Epub 2020 Apr 3. J Biomed Mater Res A. 2020. PMID: 32196926
-
Augmented bioavailability of felodipine through an α-linolenic acid-based microemulsion.Drug Deliv Transl Res. 2018 Feb;8(1):204-225. doi: 10.1007/s13346-017-0453-9. Drug Deliv Transl Res. 2018. PMID: 29204927
-
Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy.Curr Med Chem. 2018;25(25):2938-2953. doi: 10.2174/0929867324666170314143335. Curr Med Chem. 2018. PMID: 28292234 Review.
-
Microemulsions based transdermal drug delivery systems.Curr Drug Discov Technol. 2014;11(3):169-80. doi: 10.2174/157016381103141128113034. Curr Drug Discov Technol. 2014. PMID: 25466399 Review.
Cited by
-
Liposome Nanocarriers Based on γ Oryzanol: Preparation, Characterization, and In Vivo Assessment of Toxicity and Antioxidant Activity.ACS Omega. 2024 Jan 11;9(3):3554-3564. doi: 10.1021/acsomega.3c07339. eCollection 2024 Jan 23. ACS Omega. 2024. PMID: 38284009 Free PMC article.
-
Exploring the Versatility of Microemulsions in Cutaneous Drug Delivery: Opportunities and Challenges.Nanomaterials (Basel). 2023 May 21;13(10):1688. doi: 10.3390/nano13101688. Nanomaterials (Basel). 2023. PMID: 37242104 Free PMC article. Review.
References
-
- Neto A.O.W., da Silva V.L., Rodrigues D.V., Ribeiro L.S., da Silva D.N.N., Freitas J.C.D.O. A novel oil-in-water microemulsion as a cementation flushing fluid for removing non-aqueous filter cake. J. Pet. Sci. Eng. 2020;184:106536. doi: 10.1016/j.petrol.2019.106536. - DOI
-
- Dantas T.N.C., Santanna V.C., Souza T.T.C., Lucas C.R.S., Dantas Neto A.A., Aum P.T.P. Microemulsios and Nanoemulsions Applied to Well Stimulation and Enhanced Oil Recovery. Brazil. J. Petrol. Gas. 2018;12:251–265. doi: 10.5419/bjpg2018-0023. - DOI
-
- Mohammed H.A., Khan R.A., Singh V., Yusuf M., Akhtar N., Sulaiman G.M., Albukhaty S., Abdellatif A.A.H., Khan M., Mohammed S.A.A., et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev. 2023;12:20220517. doi: 10.1515/ntrev-2022-0517. - DOI
-
- Jabir M.S., Rashid T.M., Nayef U.M., Albukhaty S., AlMalki F.A., Albaqami J., AlYamani A.A., Taqi Z.J., Sulaiman G.M. Inhibition of Staphylococcus aureus α-hemolysin production using nanocurcumin capped Au@ZnO nanocomposite. Bioinorg. Chem. Appl. 2022;2022:2663812. doi: 10.1155/2022/2663812. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

