Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[ b,f]oxepine Derivatives
- PMID: 37110792
- PMCID: PMC10146957
- DOI: 10.3390/molecules28083558
Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[ b,f]oxepine Derivatives
Abstract
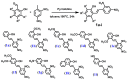
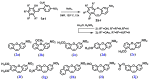
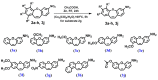
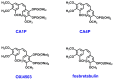
Cancer is one of the most common causes of human death worldwide; thus, numerous therapies, including chemotherapy, have been and are being continuously developed. In cancer cells, an aberrant mitotic spindle-a microtubule-based structure necessary for the equal splitting of genetic material between daughter cells-leads to genetic instability, one of the hallmarks of cancer. Thus, the building block of microtubules, tubulin, which is a heterodimer formed from α- and β-tubulin proteins, is a useful target in anti-cancer research. The surface of tubulin forms several pockets, i.e., sites that can bind factors that affect microtubules' stability. Colchicine pockets accommodate agents that induce microtubule depolymerization and, in contrast to factors that bind to other tubulin pockets, overcome multi-drug resistance. Therefore, colchicine-pocket-binding agents are of interest as anti-cancer drugs. Among the various colchicine-site-binding compounds, stilbenoids and their derivatives have been extensively studied. Herein, we report systematic studies on the antiproliferative activity of selected stilbenes and oxepine derivatives against two cancer cell lines-HCT116 and MCF-7-and two normal cell lines-HEK293 and HDF-A. The results of molecular modeling, antiproliferative activity, and immunofluorescence analyses revealed that compounds 1a, 1c, 1d, 1i, 2i, 2j, and 3h were the most cytotoxic and acted by interacting with tubulin heterodimers, leading to the disruption of the microtubular cytoskeleton.
Keywords: biological activity; cancer cell; colchicine-binding site; dibenzo[b,f]oxepine; microtubule; tubulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The stilbene and dibenzo[b,f]oxepine derivatives as anticancer compounds.Biomed Pharmacother. 2020 Mar;123:109781. doi: 10.1016/j.biopha.2019.109781. Epub 2019 Dec 19. Biomed Pharmacother. 2020. PMID: 31865147
-
Hydropathic analysis and biological evaluation of stilbene derivatives as colchicine site microtubule inhibitors with anti-leukemic activity.J Enzyme Inhib Med Chem. 2009 Dec;24(6):1237-44. doi: 10.3109/14756360902787055. J Enzyme Inhib Med Chem. 2009. PMID: 19912057 Free PMC article.
-
Synthesis, antiproliferative activity and molecular docking of thiocolchicine urethanes.Bioorg Chem. 2018 Dec;81:553-566. doi: 10.1016/j.bioorg.2018.09.004. Epub 2018 Sep 12. Bioorg Chem. 2018. PMID: 30248507
-
Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy.Biomolecules. 2022 Dec 10;12(12):1843. doi: 10.3390/biom12121843. Biomolecules. 2022. PMID: 36551271 Free PMC article. Review.
-
Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents.Curr Top Med Chem. 2017;17(22):2523-2537. doi: 10.2174/1568026617666170104145640. Curr Top Med Chem. 2017. PMID: 28056738 Review.
Cited by
-
Medicinal chemistry perspective on the structure-activity relationship of stilbene derivatives.RSC Adv. 2024 Jun 20;14(28):19823-19879. doi: 10.1039/d4ra02867h. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38903666 Free PMC article. Review.
-
Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors.Int J Mol Sci. 2024 Jun 3;25(11):6155. doi: 10.3390/ijms25116155. Int J Mol Sci. 2024. PMID: 38892342 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- Research University (IDUB) program (NChem 3)/Warsaw University of Technology
- OPUS15 2018/29/B/NZ3/02443/National Science Center
- POWR.03.02.00-00-I007/16-00 (POWER 2014-2020)/Operational Project Knowledge Education Development 2014-2020 co-financed by the European Social Fund
- Diamond Grant project/budget funds for science in the years 2019-2023
LinkOut - more resources
Full Text Sources

